HPLC Troubleshooting
Enhance your HPLC troubleshooting skills with expert-led training designed to solve common chromatographic issues. Learn to identify and fix problems related to peak shape, retention time shifts, baseline noise, pressure fluctuations, and system failures. Understand how pumps, autosamplers, detectors, and columns impact performance and how to optimize conditions for reliable results. Ideal for professionals in pharmaceuticals, biotechnology, food safety, and environmental analysis, this training includes real-world case studies, interactive quizzes, and expert insights. Master HPLC troubleshooting today and ensure consistent, high-quality separations!
HPLC Troubleshooting Masterclass
In this webcast, our speakers demonstrate practical HPLC separation troubleshooting. Using real world chromatograms submitted by CHROMacademy members from many different application areas, they illustrate how to recognize problems and explain further tests and investigations necessary to properly qualify the issue.

HPLC Troubleshooting - Autosampler, Column, and Detector Issues
In this webcast, our speakers present practical troubleshooting and maintenance information associated with HPLC autosamplers, columns, and detectors.
Troubleshooting Your HPLC Chromatogram - Selectivity, Resolution, and Baseline Issues
This session examines the common causes of changes in selectivity, loss of chromatographic resolution, and baseline issues – such as splitting, fronting, tailing, and shouldering. Strategies for problem identification and calculation of critical chromatography performance indicators will also be discussed.
HPLC Troubleshooting - Eluents and Solvent Delivery Systems
In this session, our speakers describe practical troubleshooting and maintenance information around HPLC eluents and solvent delivery systems (pumps). This session considers important practical concepts such as good practice for eluent preparation, filtration and degassing as well as common related issues such as retention time drift and selectivity changes.
Effective HPLC Troubleshooting
Troubleshooting is an essential skill for all HPLC users. By looking at the top 5 problems submitted to the CHROMacademy troubleshooter we will provide you with the fundamentals of troubleshooting which can be applied to any problem you encounter.
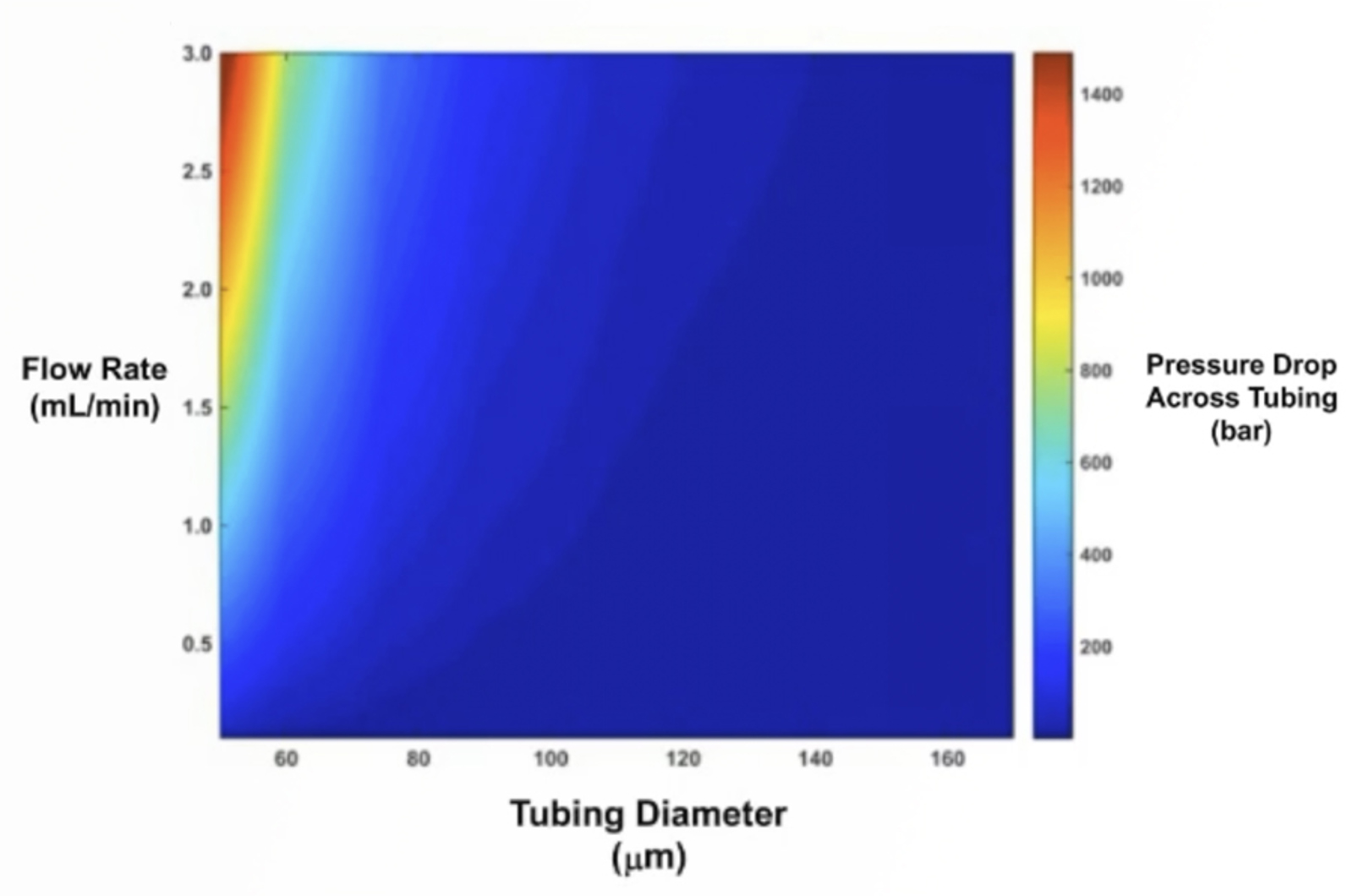
UHPLC Troubleshooting - How U is Your UHPLC?
Moving from conventional HPLC systems to UHPLC is not without its problems and the requirement to adjust to a new way of working. This webcast discusses fundamental practices such as careful sample and solvent preparation, the use of appropriate fittings and tubing, pressure/selectivity effects, the practical implication of frictional heating, and instrument parameters such as system solvent wash out volume (as opposed to gradient dwell volume). Initial conditions for method development in UHPLC and some simple, easy to use equations which can be utilized to facilitate method transfer from HPLC to UHPLC will be provided.
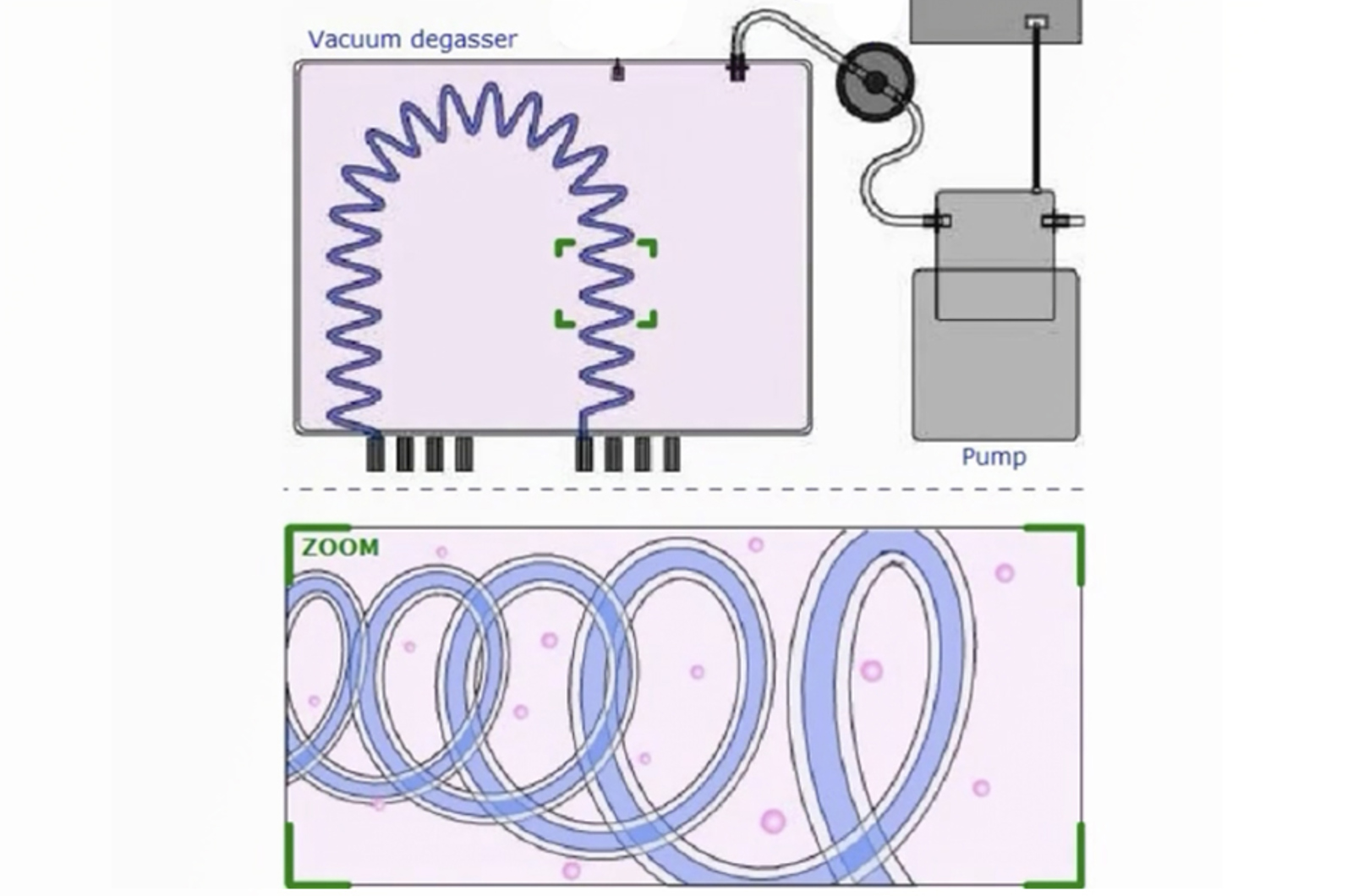
Optimize and Troubleshoot Your HPLC System: Degassers, Pumps, and Autosamplers
Learn how HPLC instrument components function in order to correctly maintain and troubleshoot the system for maximum up-time and optimum chromatography. This webcast will focus on the degasser, pumps, and autosampler. Poorly degassed solvents will cause noisy baselines and columns may be damaged; pumps which do not deliver the correct flow rate can result in drifting retention times; while an autosampler which does not deliver the correct sample volume will impact peak shape and quantitation.
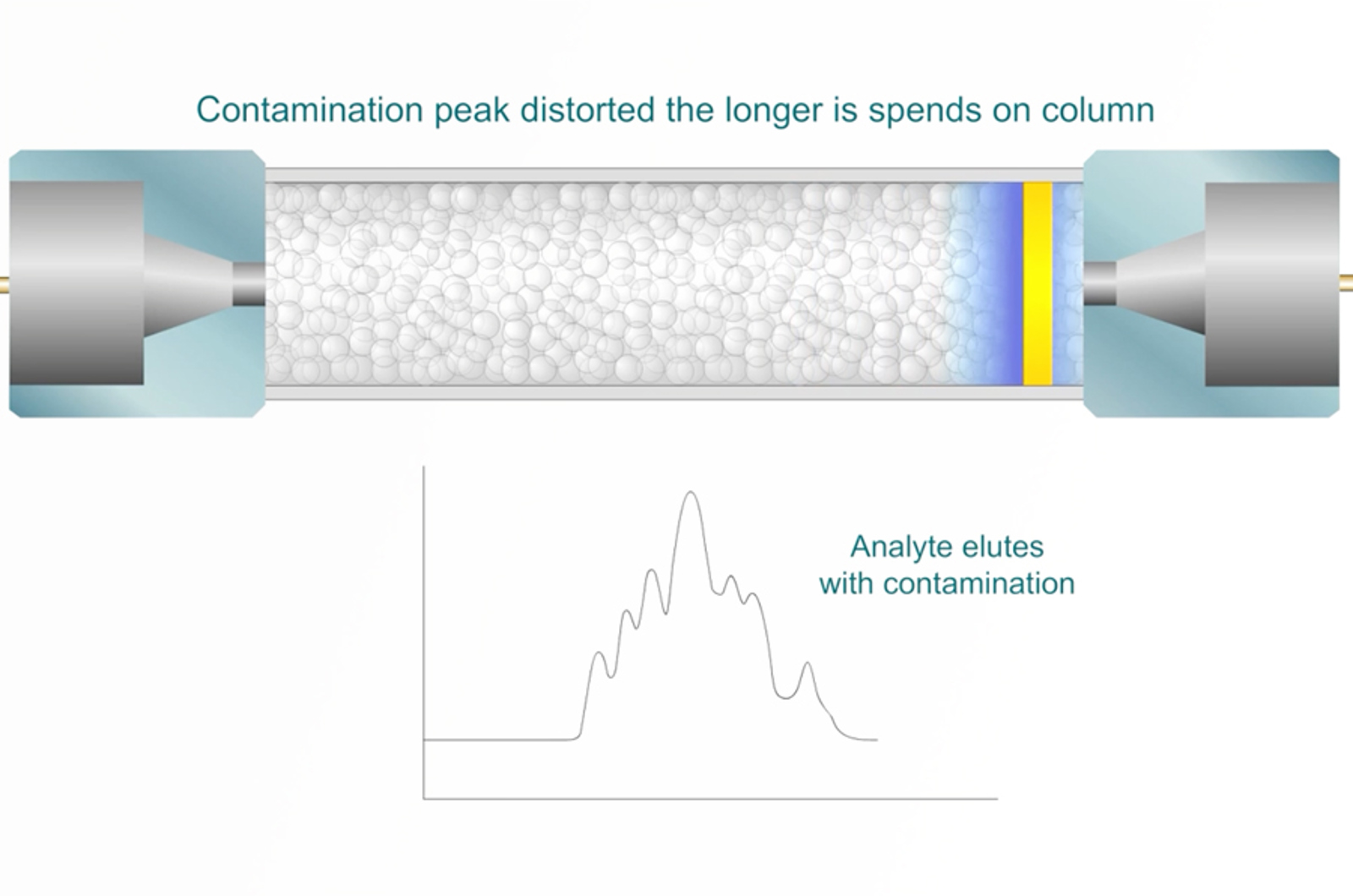
Troubleshooting Extra Peaks in HPLC
In this module we’re going to take a look at a very common problem in HPLC – extra peaks and contamination. We’ll look at the main types of contamination, the potential sources, defining the problem and main types of investigational re-analysis. The module is supplemented by real life case study workshops, in which you have a virtual HPLC that allows you to select what additional investigations you’d like to perform.
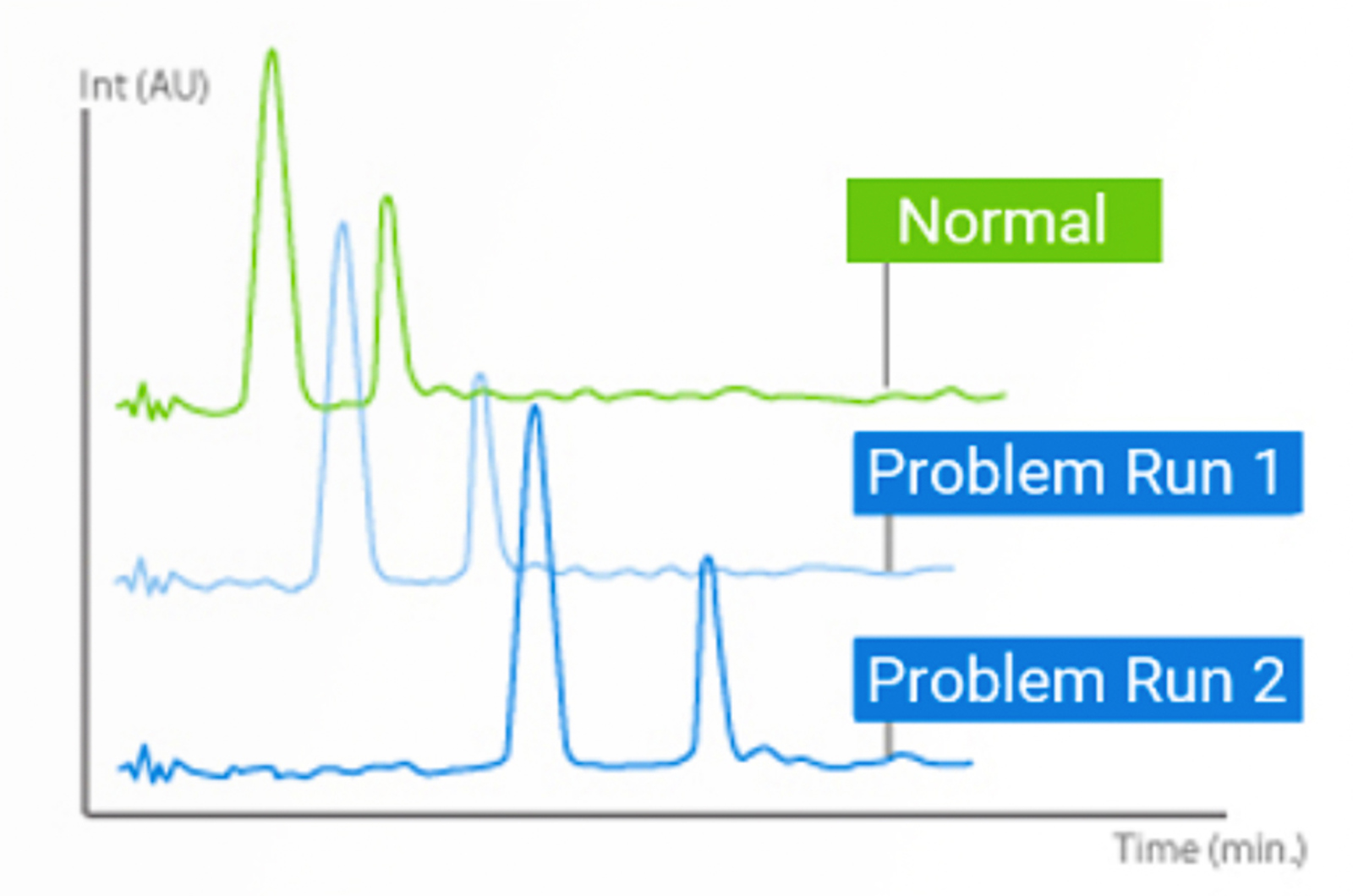
Retention Time Variability in HPLC
There are a whole bunch of retention time issues that cause problems in HPLC. A lot of the underlying causes we can do something about – others we just need to be aware of the cause and put our minds to rest. This quick guide will outline how to overcome or better control these issues.
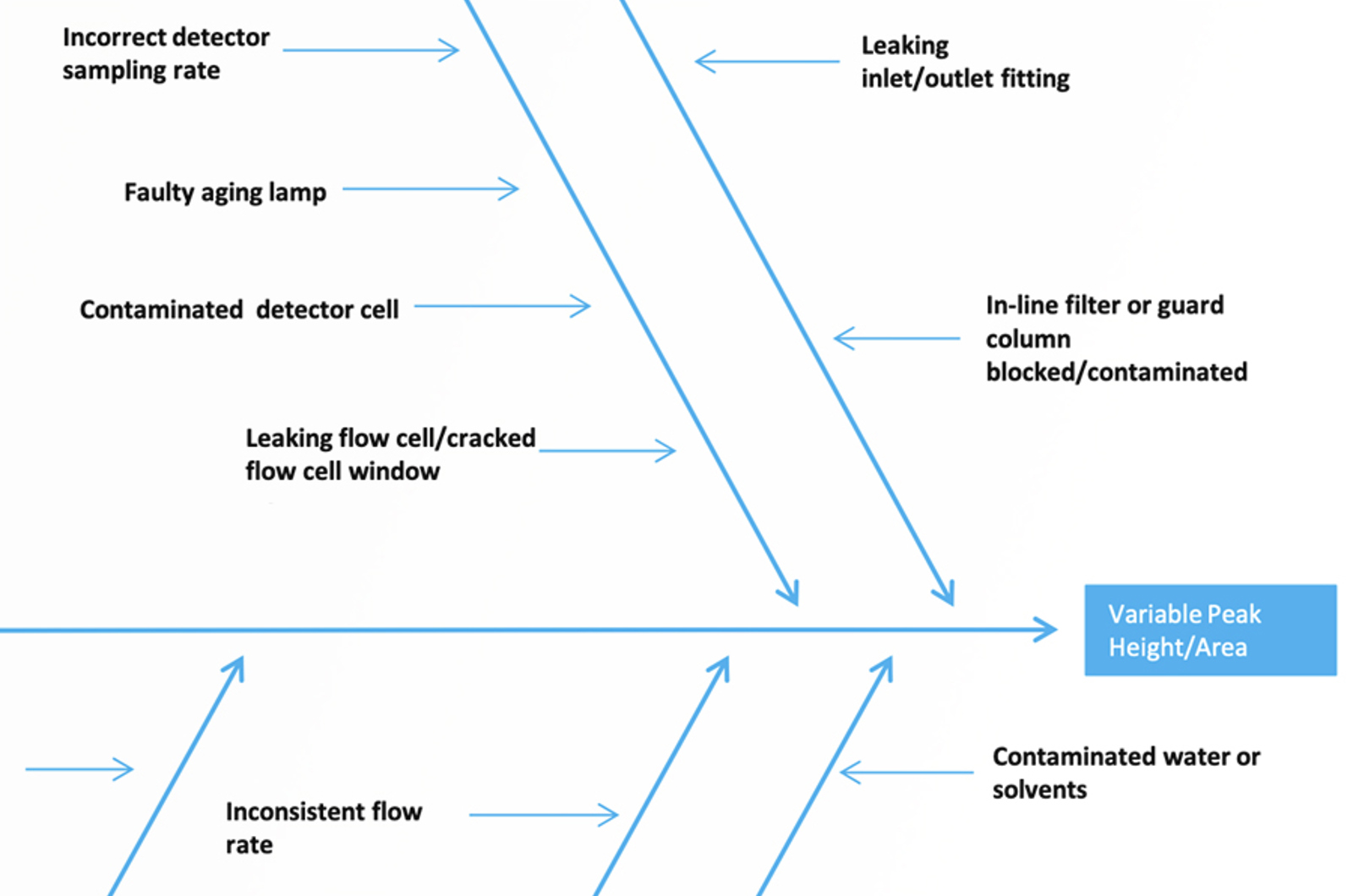
An Alternative Approach to Troubleshooting Variable Peak Height/Area in HPLC
The problem of variable peak height/area is a common and complex one, with many potential causes. We have constructed an Ishikawa diagram to look at the possible causes of variable peak height/area and to help prioritize the approach to fixing the problem.

HPLC Troubleshooter
We developed the CHROMacademy HPLC troubleshooter with busy chromatographers in mind. In 3 simple steps we can help you overcome your instrument, separation, and quantitation issues.
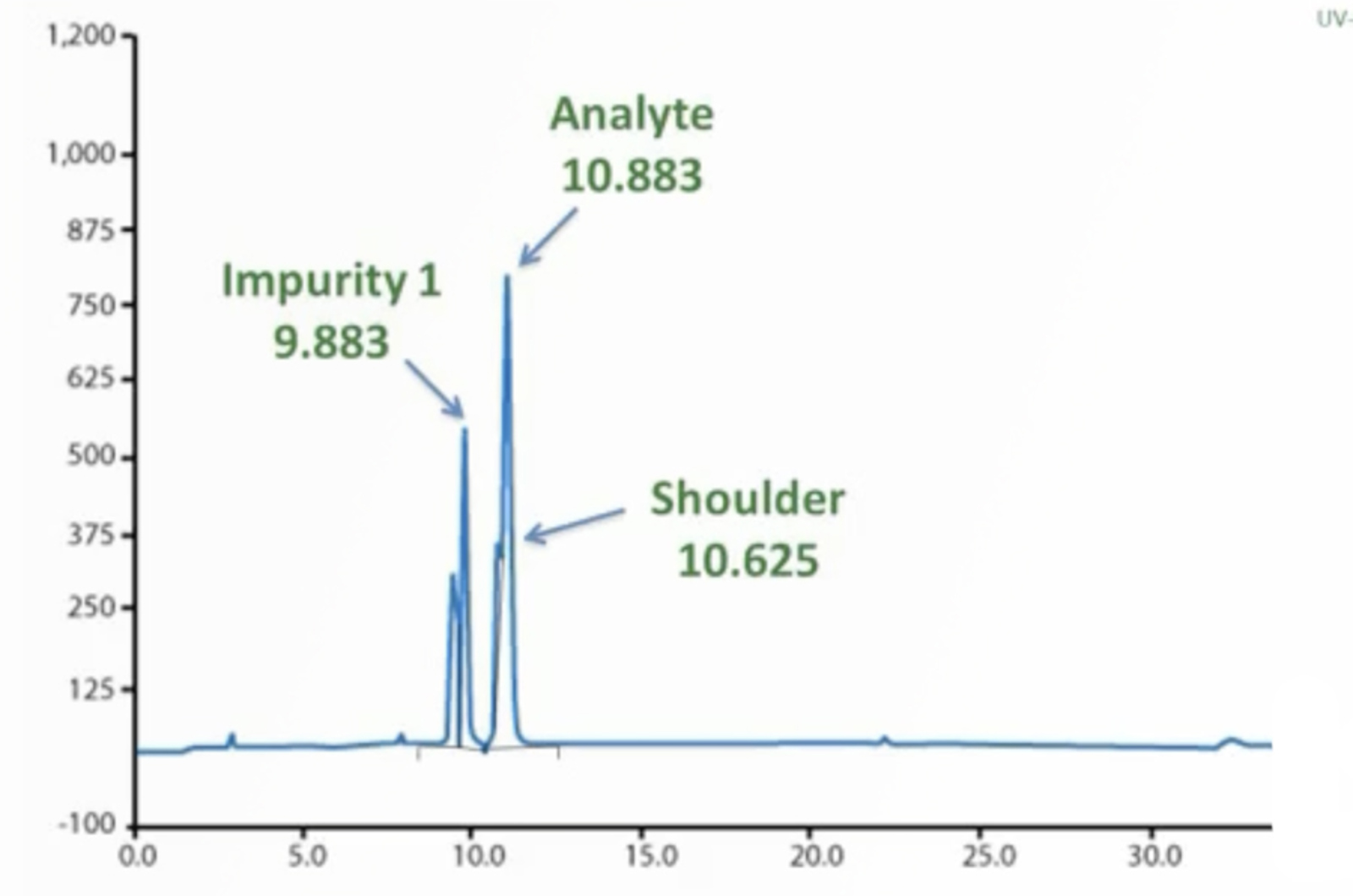
HPLC Method Troubleshooting
Troubleshooting is a task that all chromatographers must undertake routinely. Some problems seem to be resolved quickly while others can be much more onerous. Having a good troubleshooting strategy which can be applied to all problems is essential, therefore, using examples of real-life chromatographic problems we will provide you with the knowledge you need to become the expert troubleshooter in your lab.
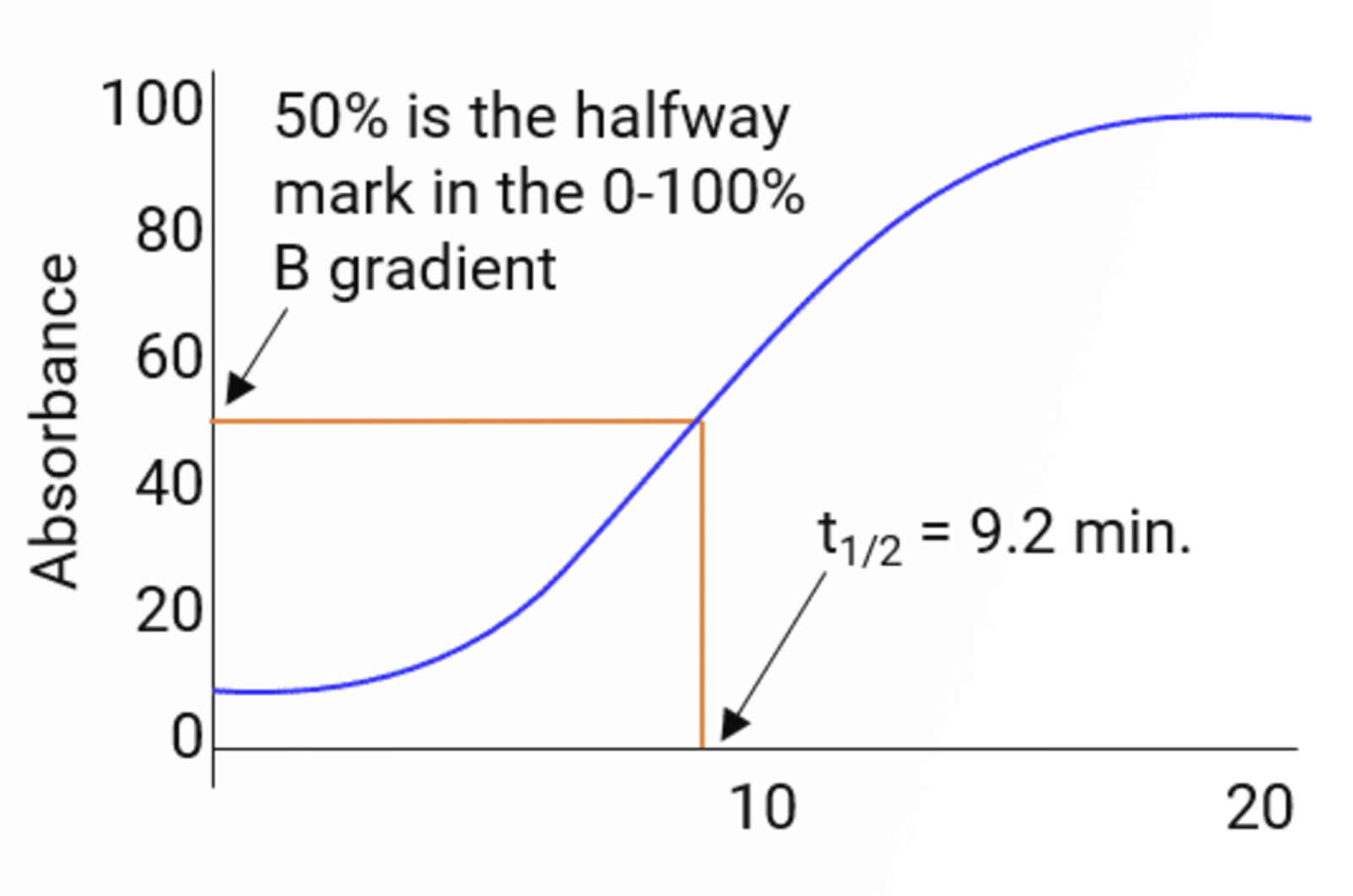
HPLC Gradient Elution – Baseline Drift
This quick guide provides information on how to avoid drifting baselines when using gradient elution. These include the use of reference wavelengths and absorbance matching.
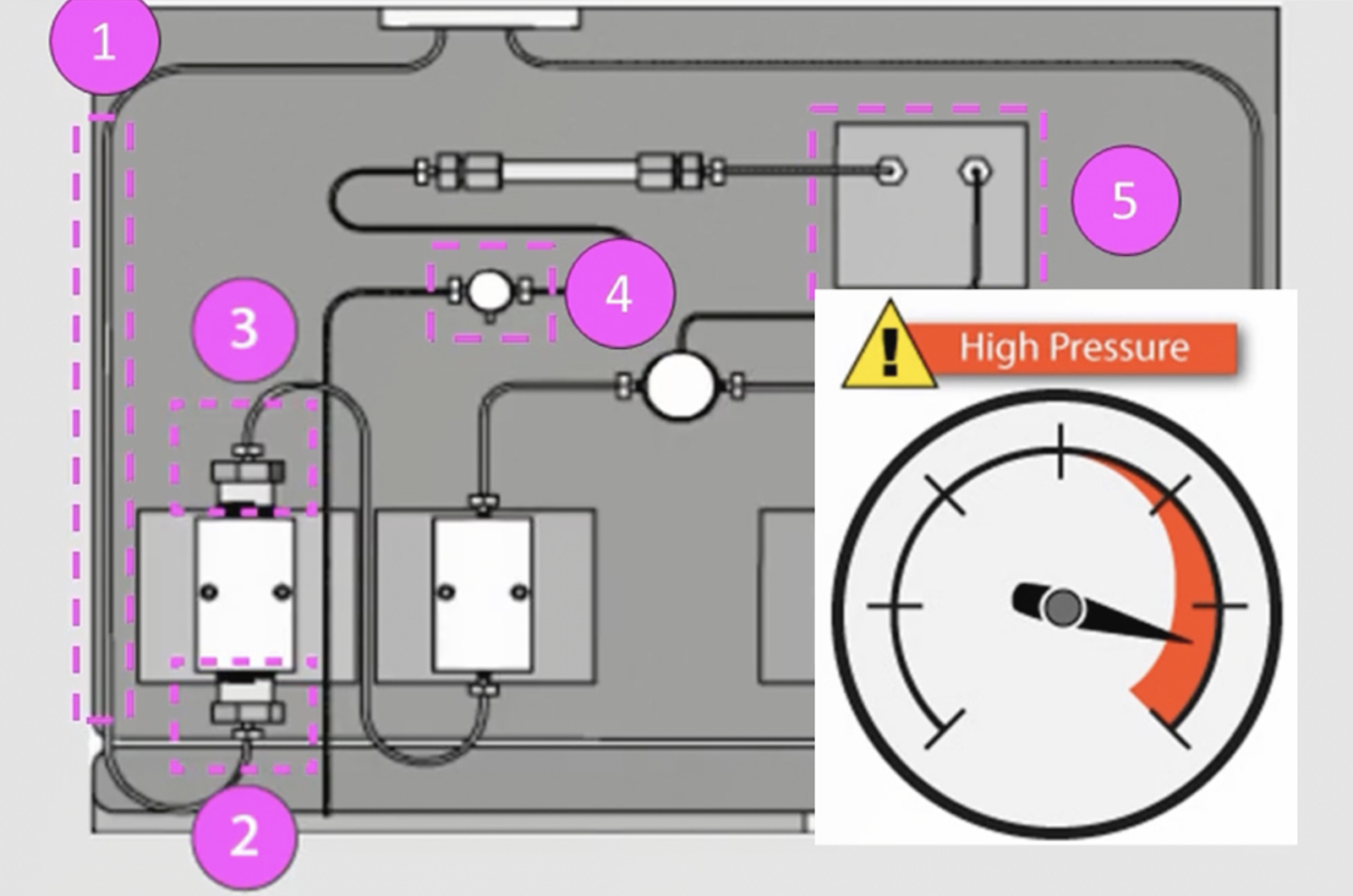
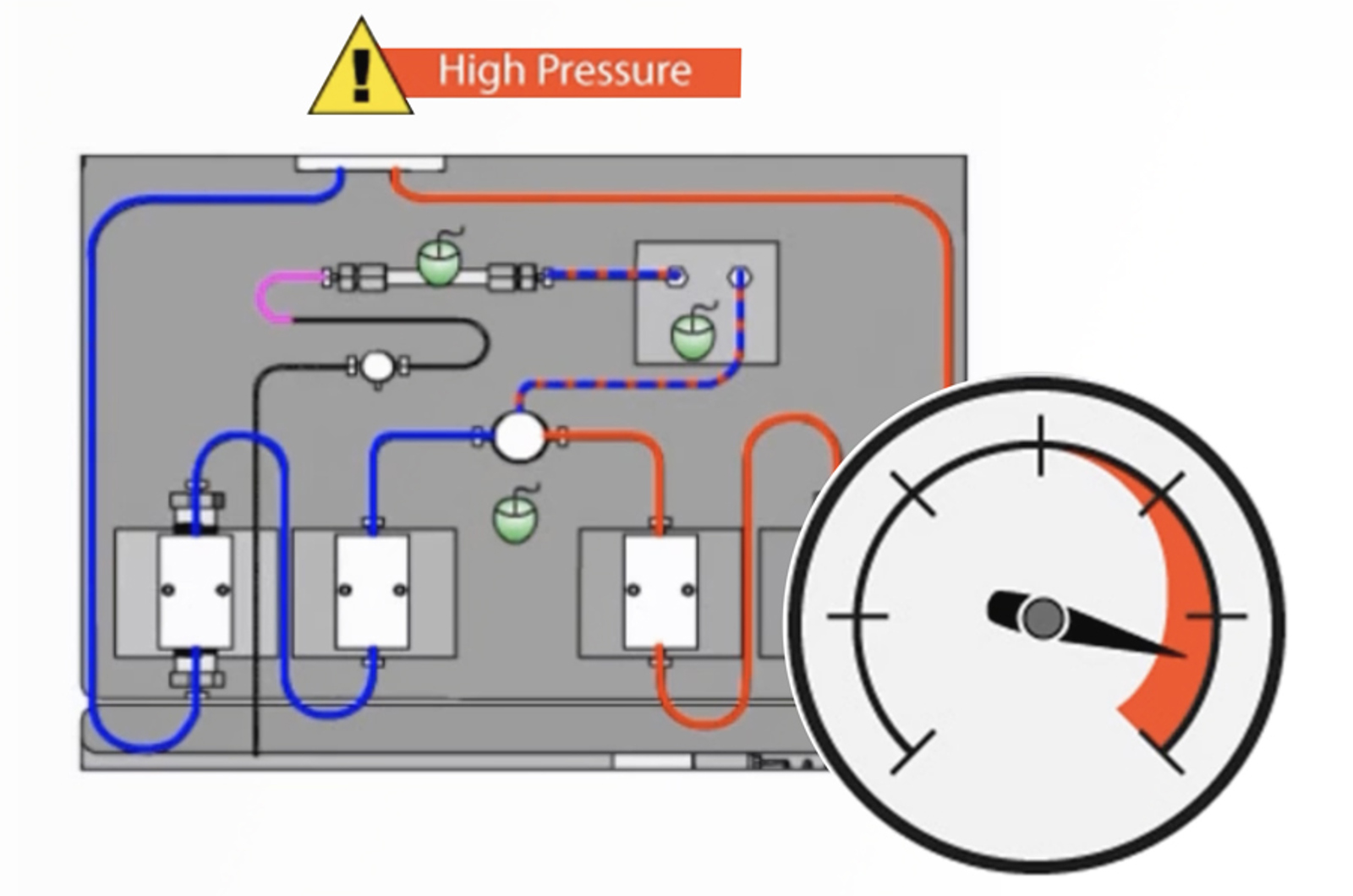
HPLC Troubleshooting Video Guide to High Pump Backpressure
High backpressure in the system results in the pump working under greater resistance which will lead to an increase in the need for maintenance. Learn how to locate the source of high backpressure in your HPLC system and resolve the problem.
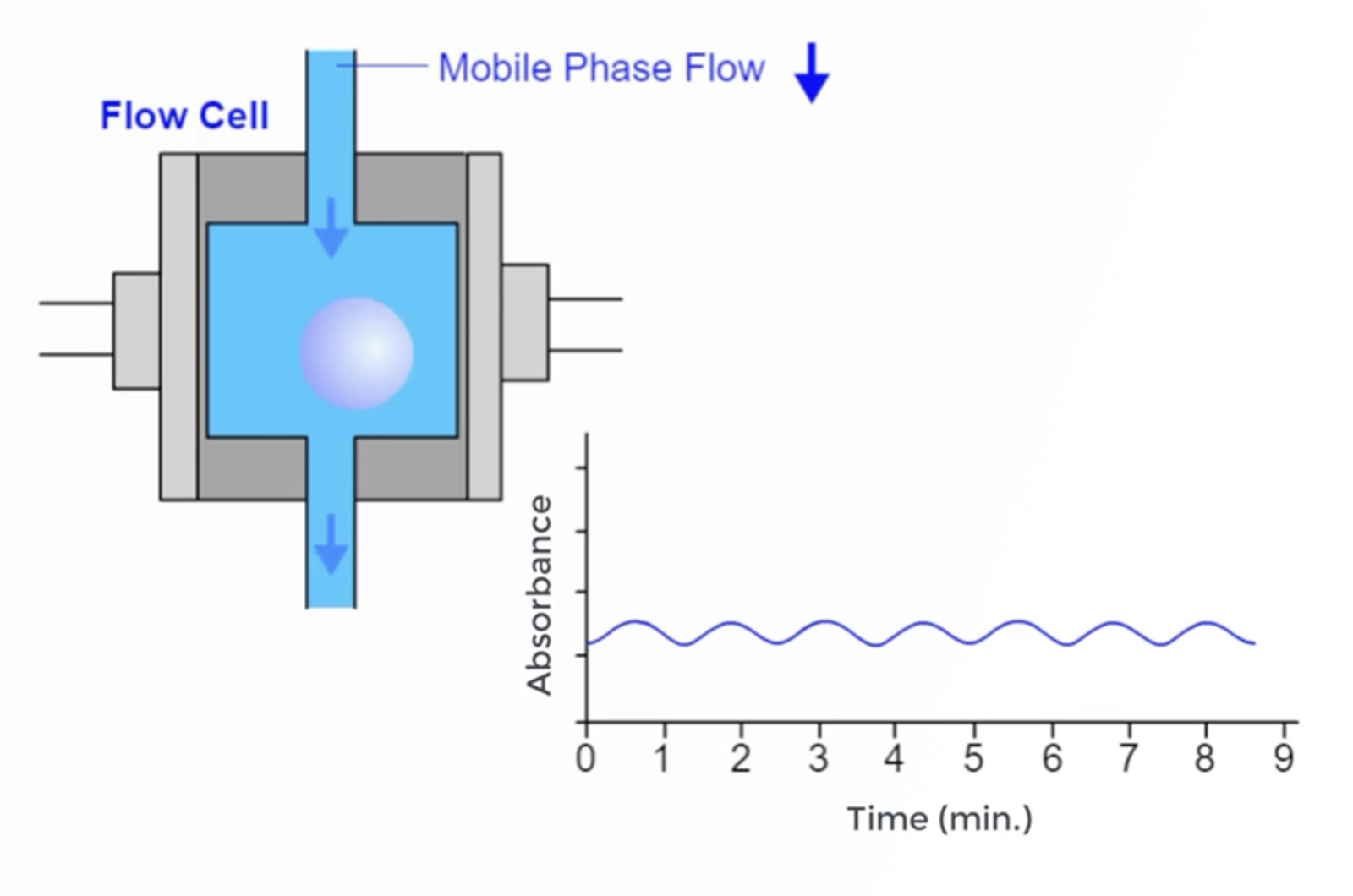
Practical HPLC Troubleshooting Video - Cycling Baseline
In this video, we solve a cycling HPLC baseline problem submitted to our Ask the Expert service by a CHROMacademy member. This video shows how to use the interactive HPLC Troubleshooter to identify potential causes for the problem. We then discuss each of the potential causes, how they might affect the appearance of our baseline, and what to do to avoid the problems happening again.
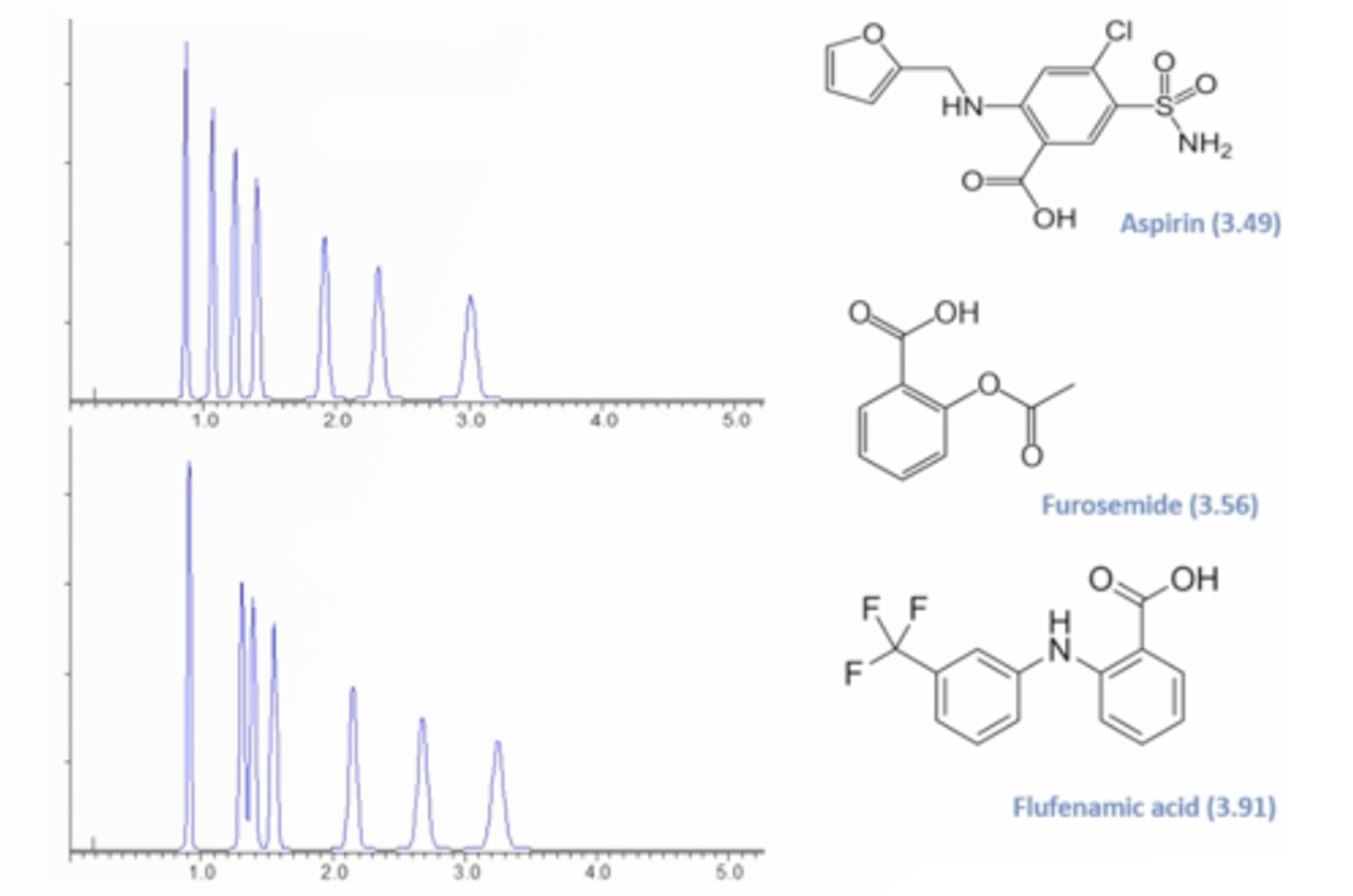
Practical HPLC Troubleshooting Video - Retention Time and Selectivity
This examples solves an intractable problem of a fast HPLC-MS separation which shows small but disruptive changes in retention time and selectivity for a number of ionizable compounds. The CHROMacademy Troubleshooter is used to diagnose the problem(s) after which we explain how to avoid problems with robustness when using eluent additives which are compatible with mass spectrometric detection.
Chromatography Troubleshooting Tips
These 10 tips can be used to help you start troubleshooting any HPLC problem.
HPLC Troubleshooting Video - Peak Tailing
Tailing peaks create issues with resolution, quantitation (integration), and reproducibility. Peak shape is often the controlling factor when optimizing complex separations, especially when components are present in very different concentrations. Therefore, we are going to look at the top 5 causes of peak tailing provided by our HPLC Troubleshooter – these results have been ranked so are the most likely causes of peak tailing and should, therefore, be your first consideration when you are faced with this problem.
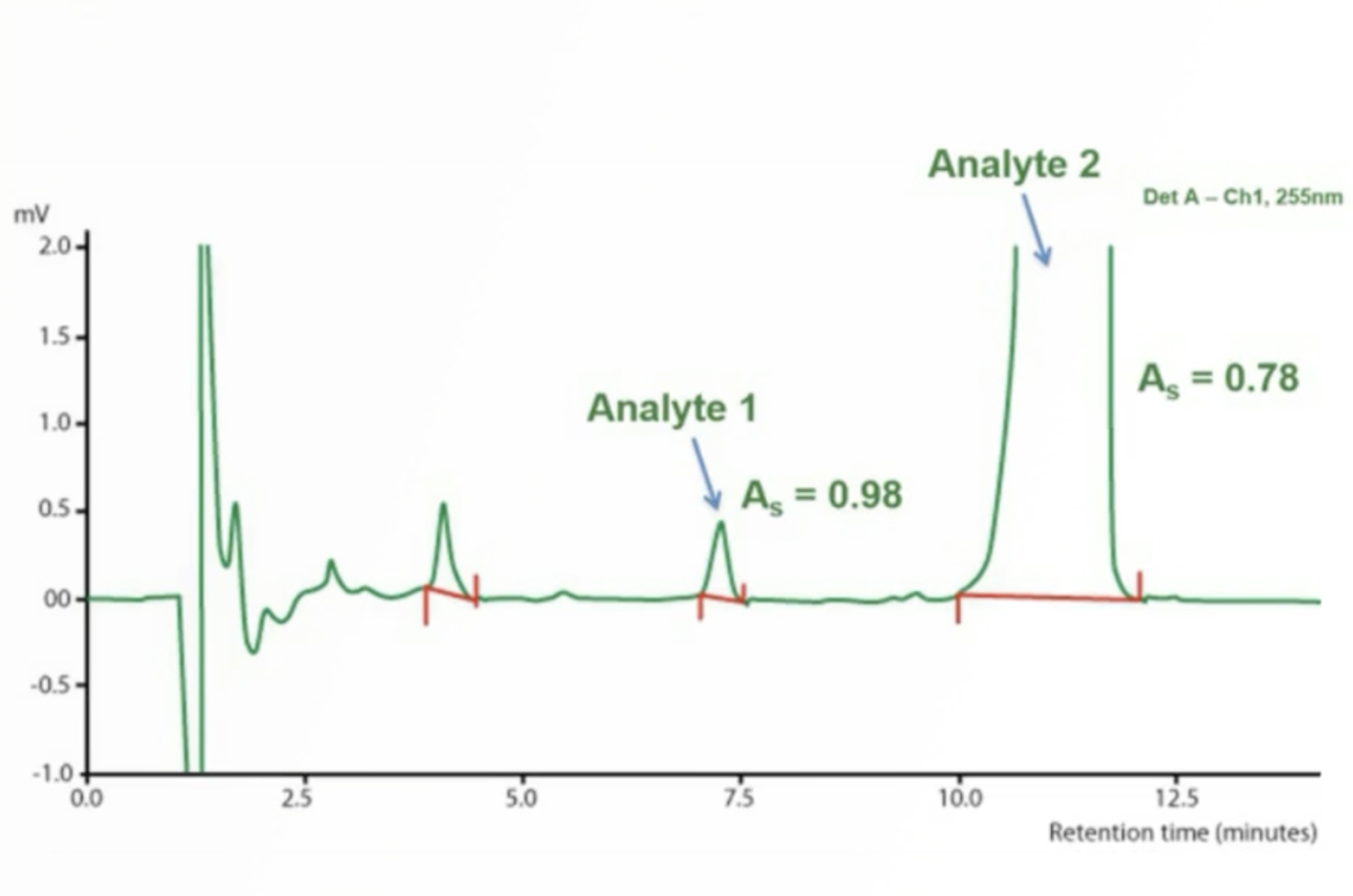
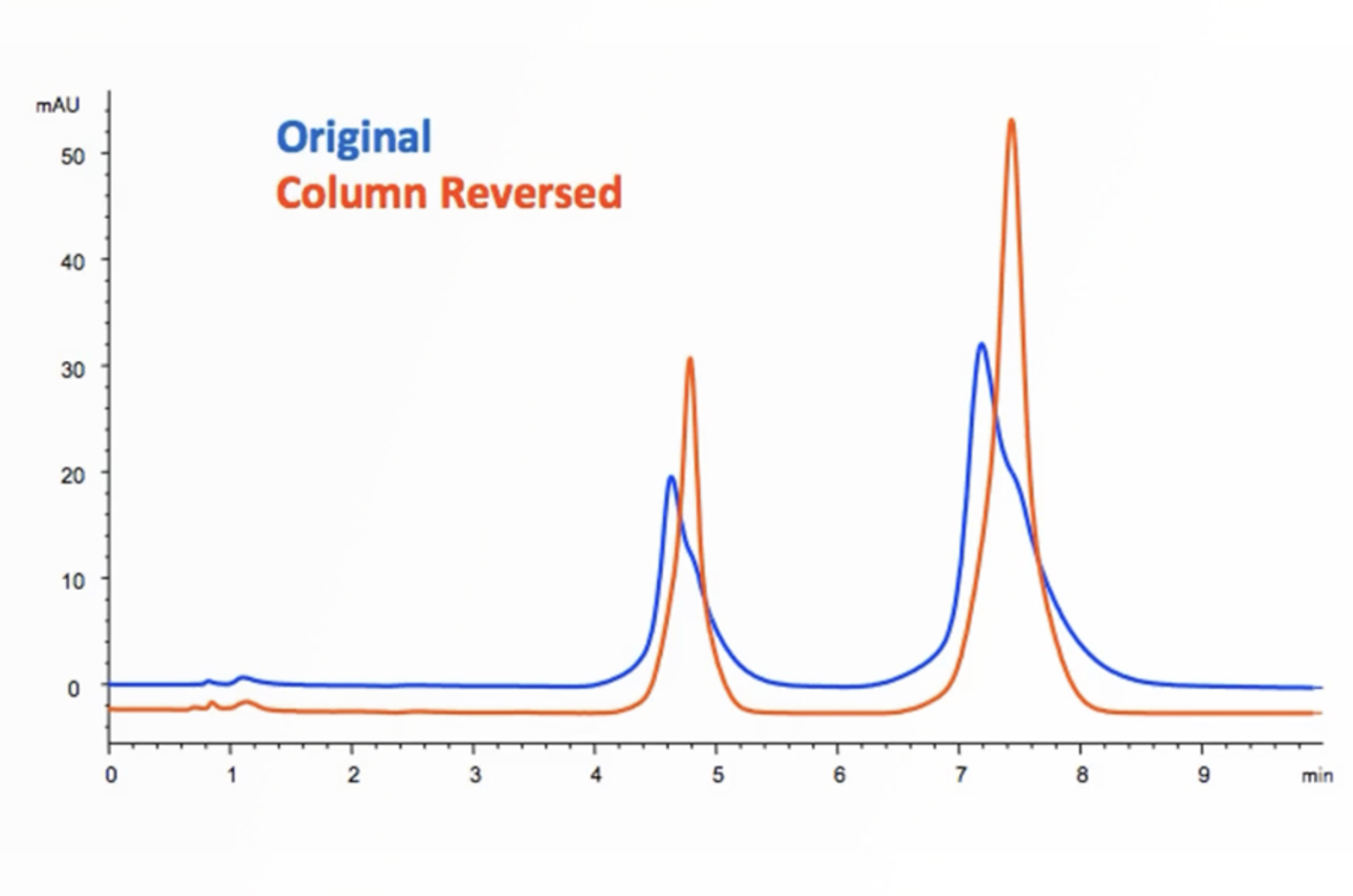
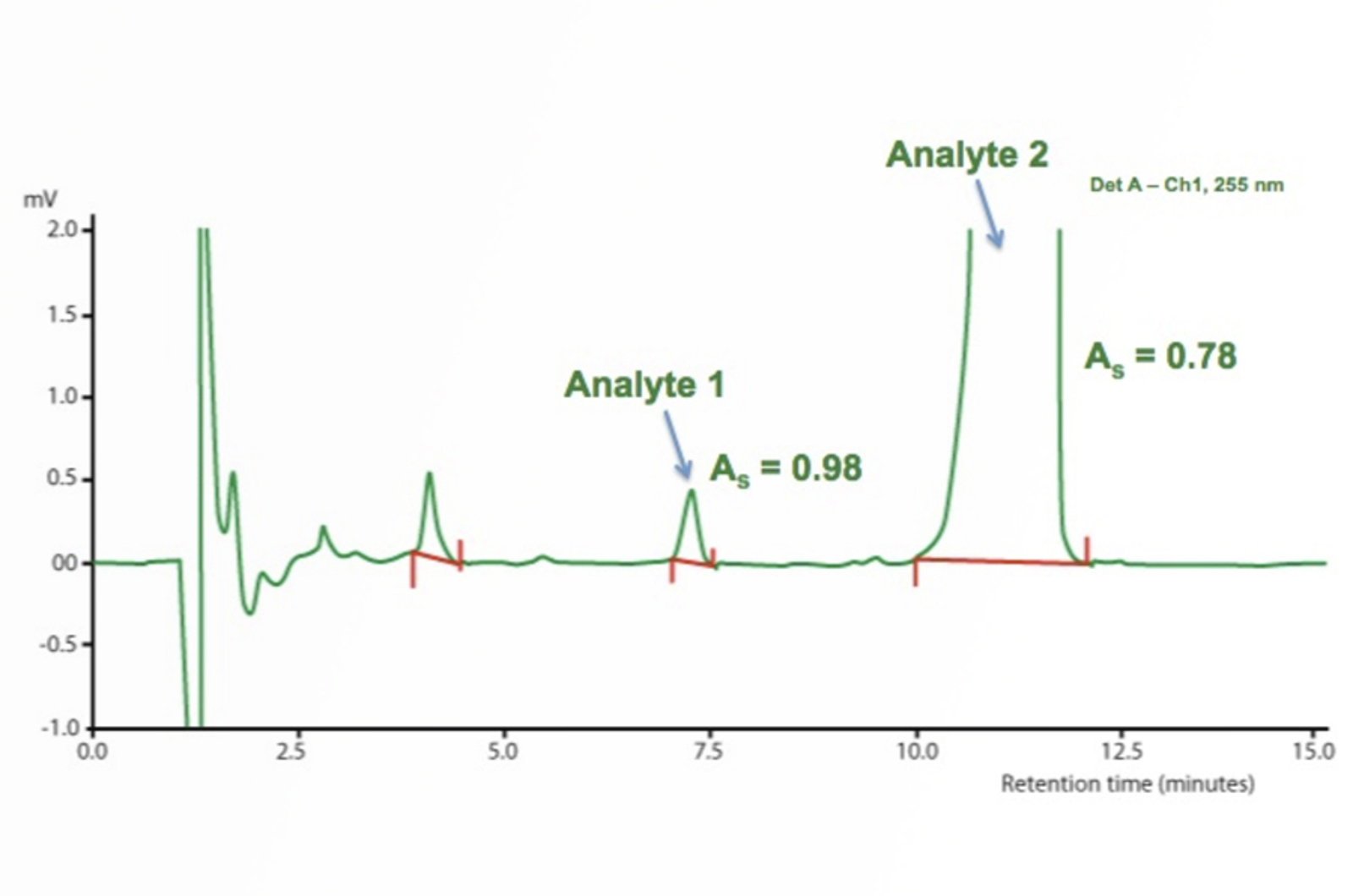
HPLC Troubleshooting - More Split Peaks
Peak splitting can be caused by either physical or chemical issues – asking the right questions and knowing the common causes of peak splitting will help to quickly troubleshoot the problem. The real-life example in this quick guide will show you how.
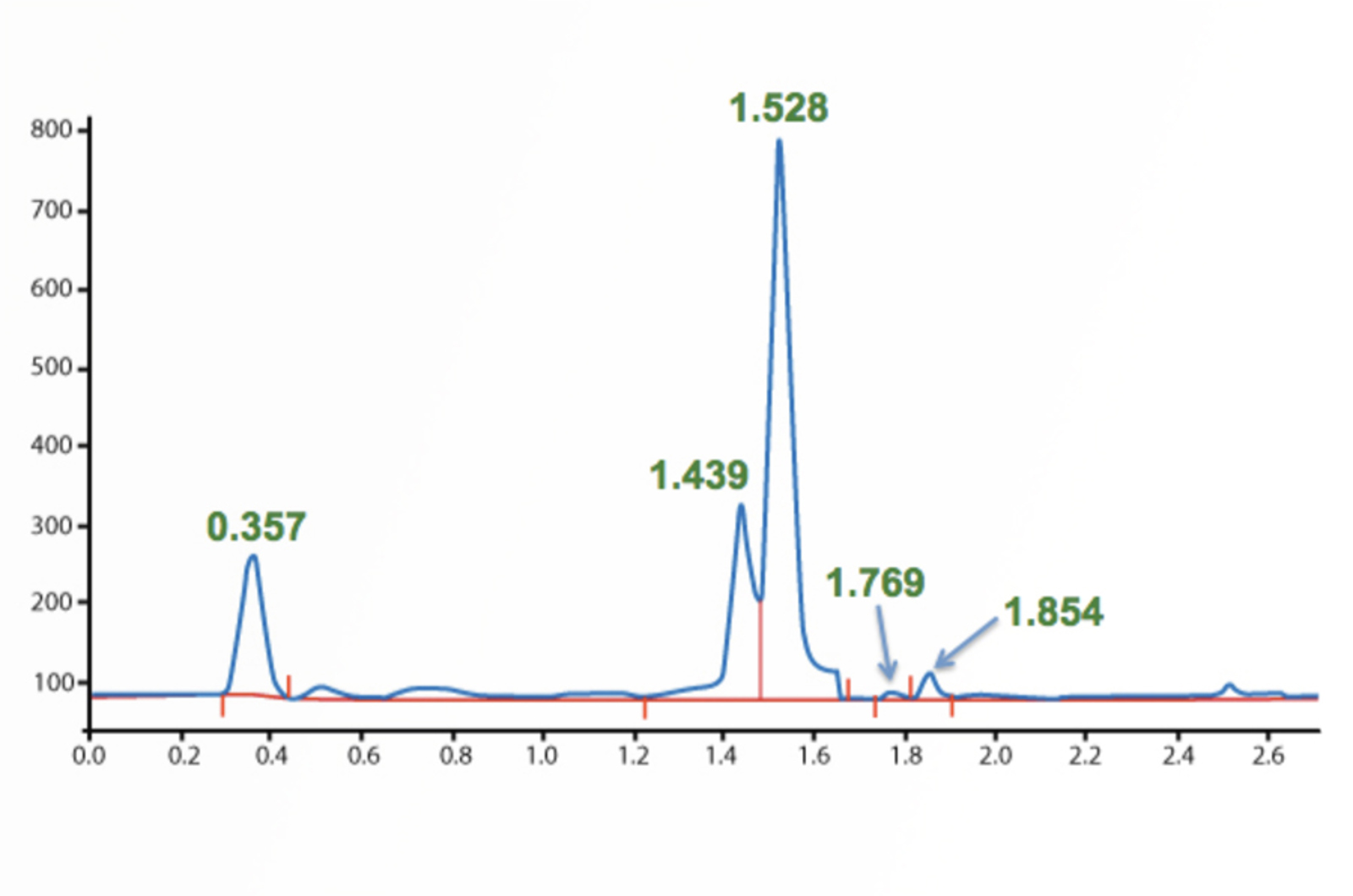
HPLC Troubleshooting – Retention Time and Baseline Issues
This troubleshooting example investigates the reasons behind some strange retention time and baseline position shifts in the analysis of a glycoprotein dimer.
HPLC Troubleshooting Guide to Peak Fronting and Poor Sensitivity
Using a real world problem this quick guide will show you how to recognize the problem of peak fronting and the steps which should be taken during troubleshooting.
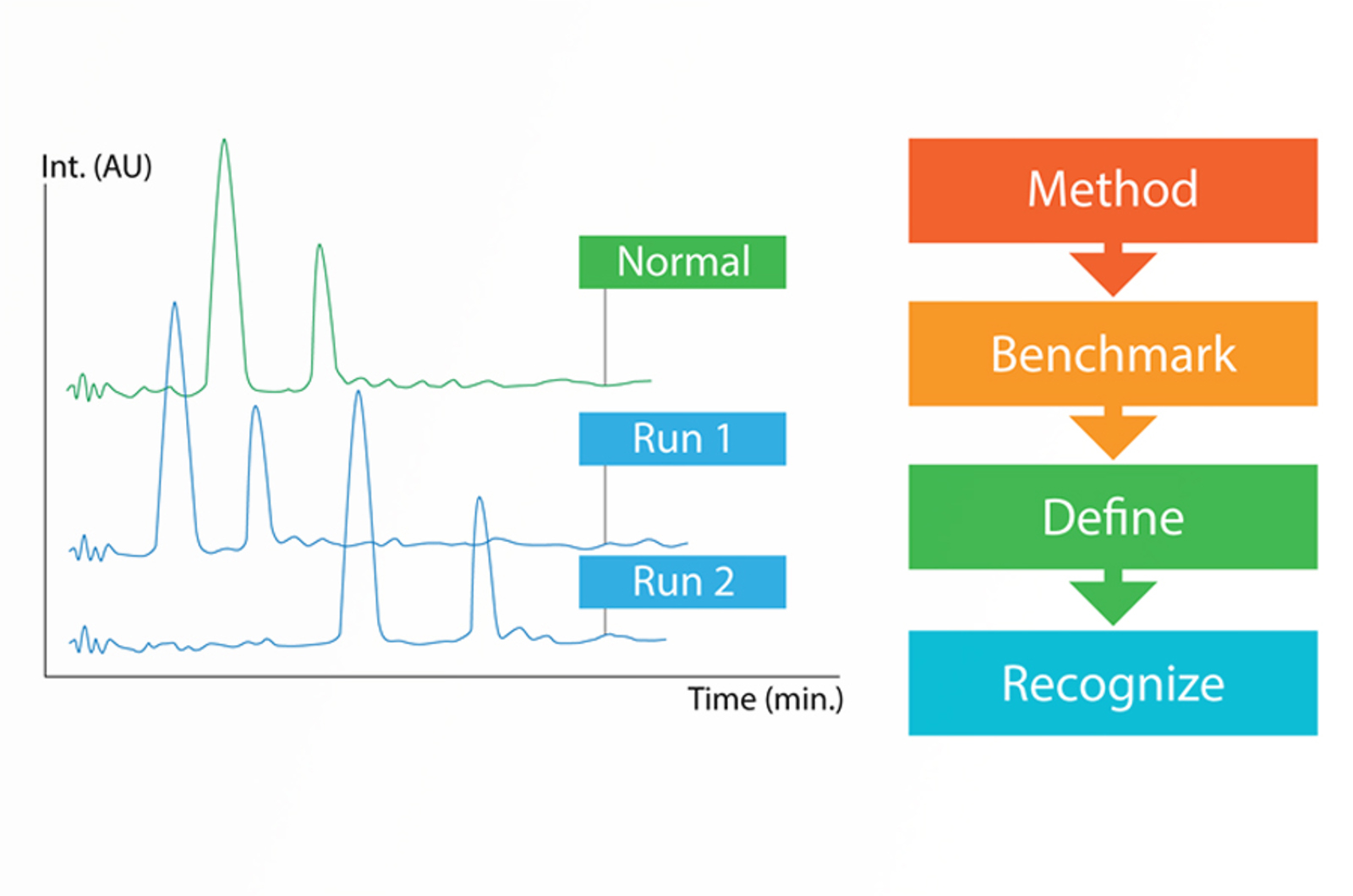
HPLC Troubleshooting Strategy
This five step troubleshooting strategy will set you up for success when troubleshooting any HPLC problem.
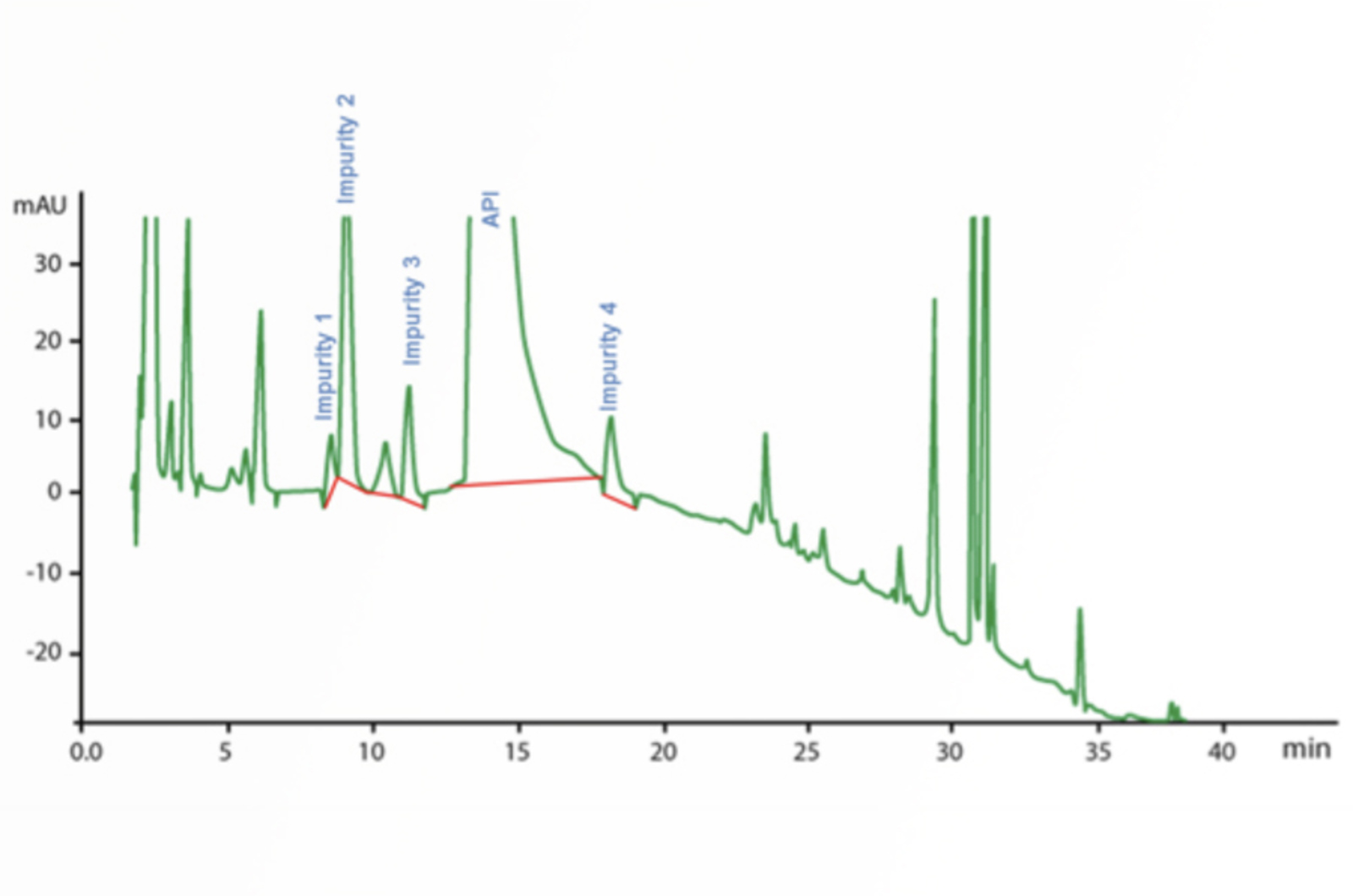
HPLC Troubleshooting Guide to Changes in Selectivity, Retention, and Resolution
This article gives a guide for troubleshooting changes in selectivity, retention, and resolution.
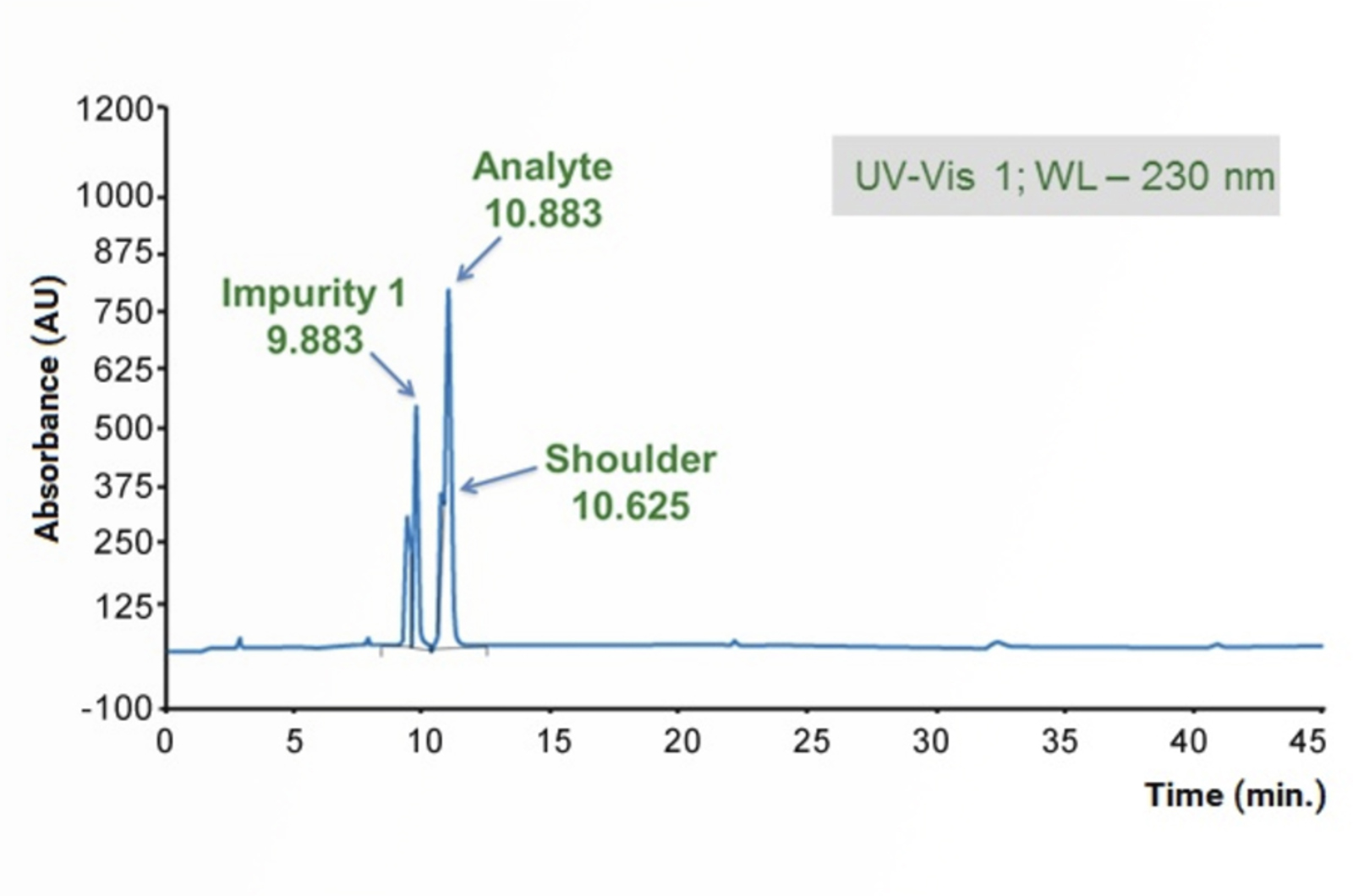
HPLC Troubleshooting Guide to Peak Splitting Problems
This quick guide focuses on troubleshooting the most common causes of peak splitting problems using the CHROMacademy interactive HPLC Troubleshooter.
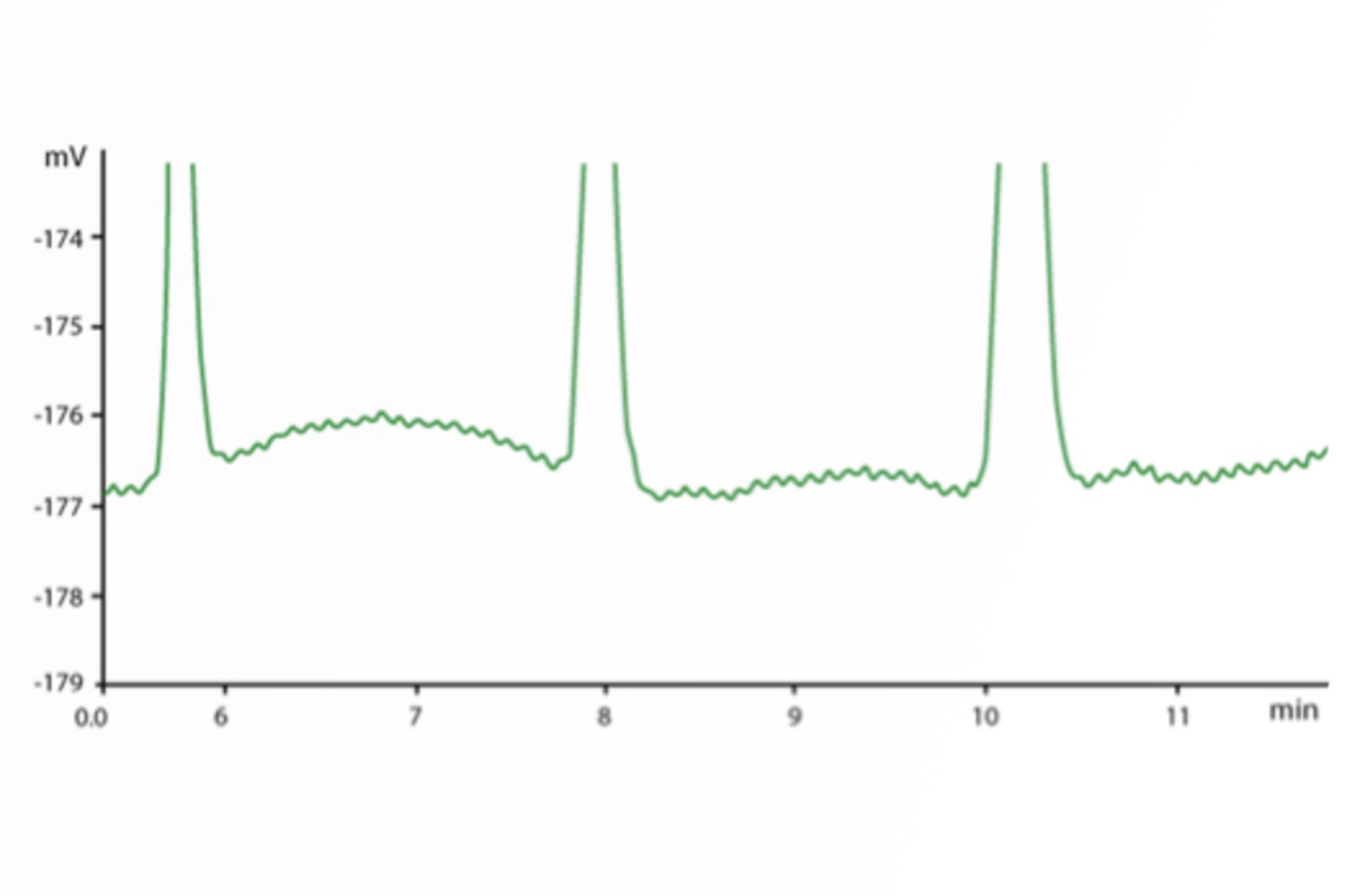
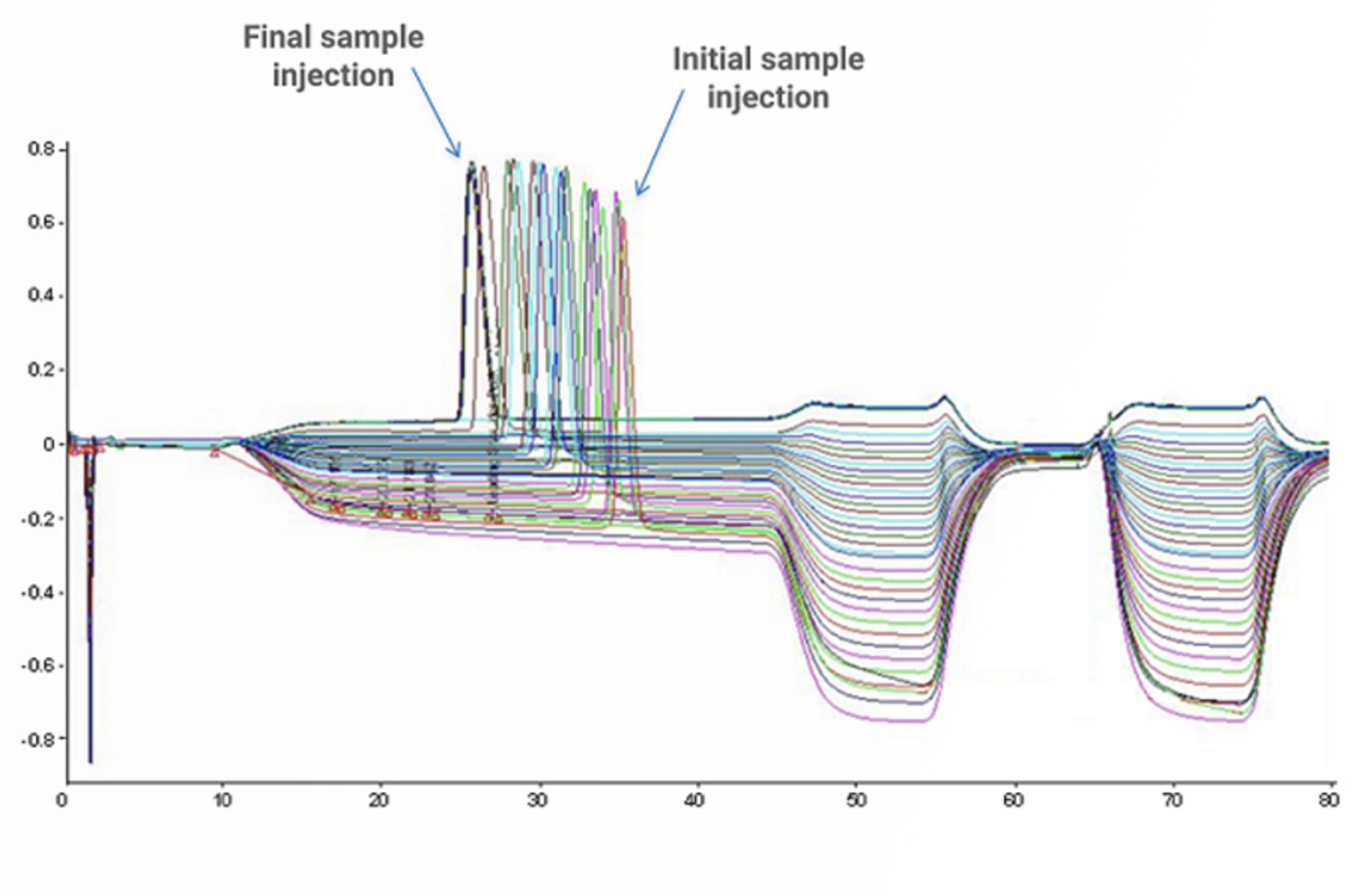
HPLC Troubleshooting Guide to Cycling Baselines and Pressure Fluctuations
This quick guide focuses on troubleshooting cycling baselines and pressure fluctuations using the CHROMacademy interactive troubleshooter.
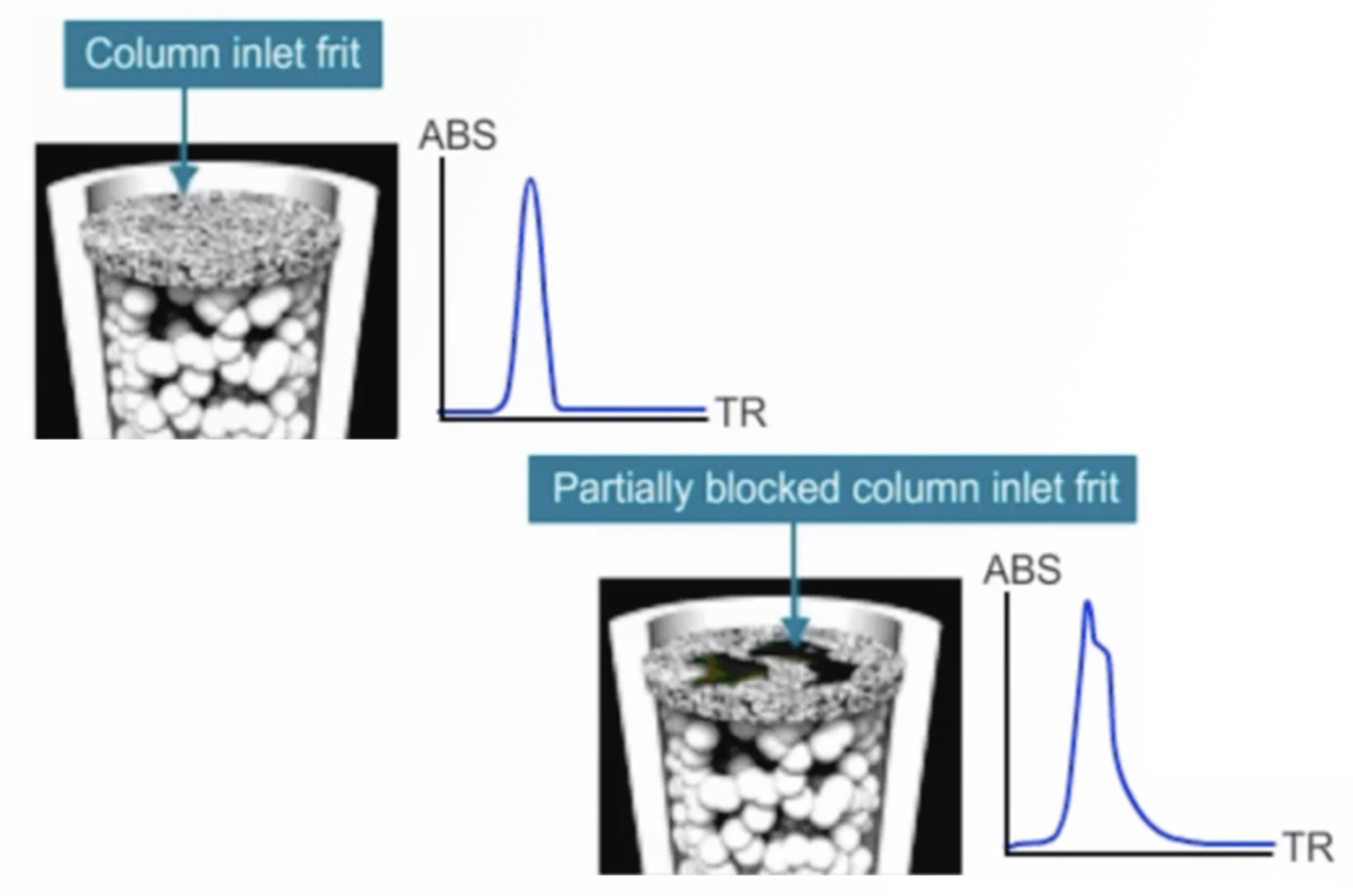
HPLC Troubleshooting – Split Peaks
Split peaks in HPLC are one of the most common problems we get asked about at CHROMacademy. With numerous causes related to both the instrument and method it can be a potentially complex problem. Watch this troubleshooting video to see how the CHROMacademy HPLC Troubleshooter can help to quickly solve this problem... and pick up a few extra troubleshooting tips too.
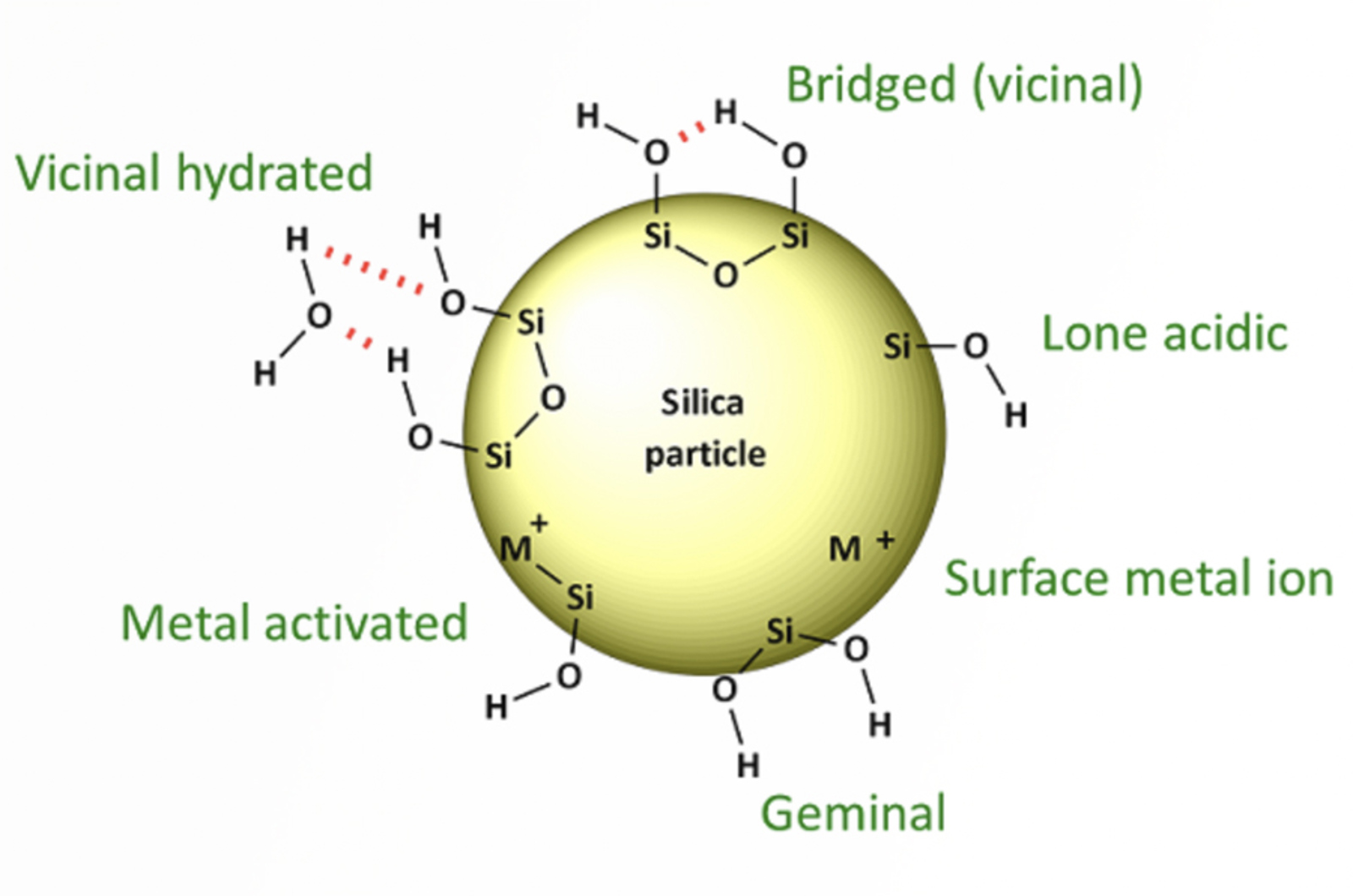
HPLC Troubleshooting Guide - Peak Tailing
Tailing peaks create issues with resolution, quantitation (integration), and reproducibility. Peak shape is often the controlling factor when optimizing complex separations, especially when components are present in very different concentrations. This article will focus on troubleshooting peak tailing.

Troubleshooting HPLC Method Reliability
Reliable methods are essential to produce data we can trust. Whether you are designing methods or transferring methods from another lab, this training will ensure that you know which areas of the HPLC method are likely to cause the most problems allowing you to successfully troubleshoot method related problems.

HPLC Column Issues
The column is at the heart of the HPLC separation, therefore, column related issues can have a big impact on the reliability of our method. We will consider the most common sources of common related issues, allowing you to troubleshoot problematic methods as well as avoiding many of these pitfalls in the first place.

Troubleshooting HPLC Mobile Phase Issues
The HPLC mobile phase impacts retention and selectivity of analytes, therefore, mistakes in designing and preparing mobile phases will lead to considerable problems. Let us teach you which areas of mobile phase preparation and design are most likely to result in method errors, ensuring you have the tools to recognize problems before they occur.
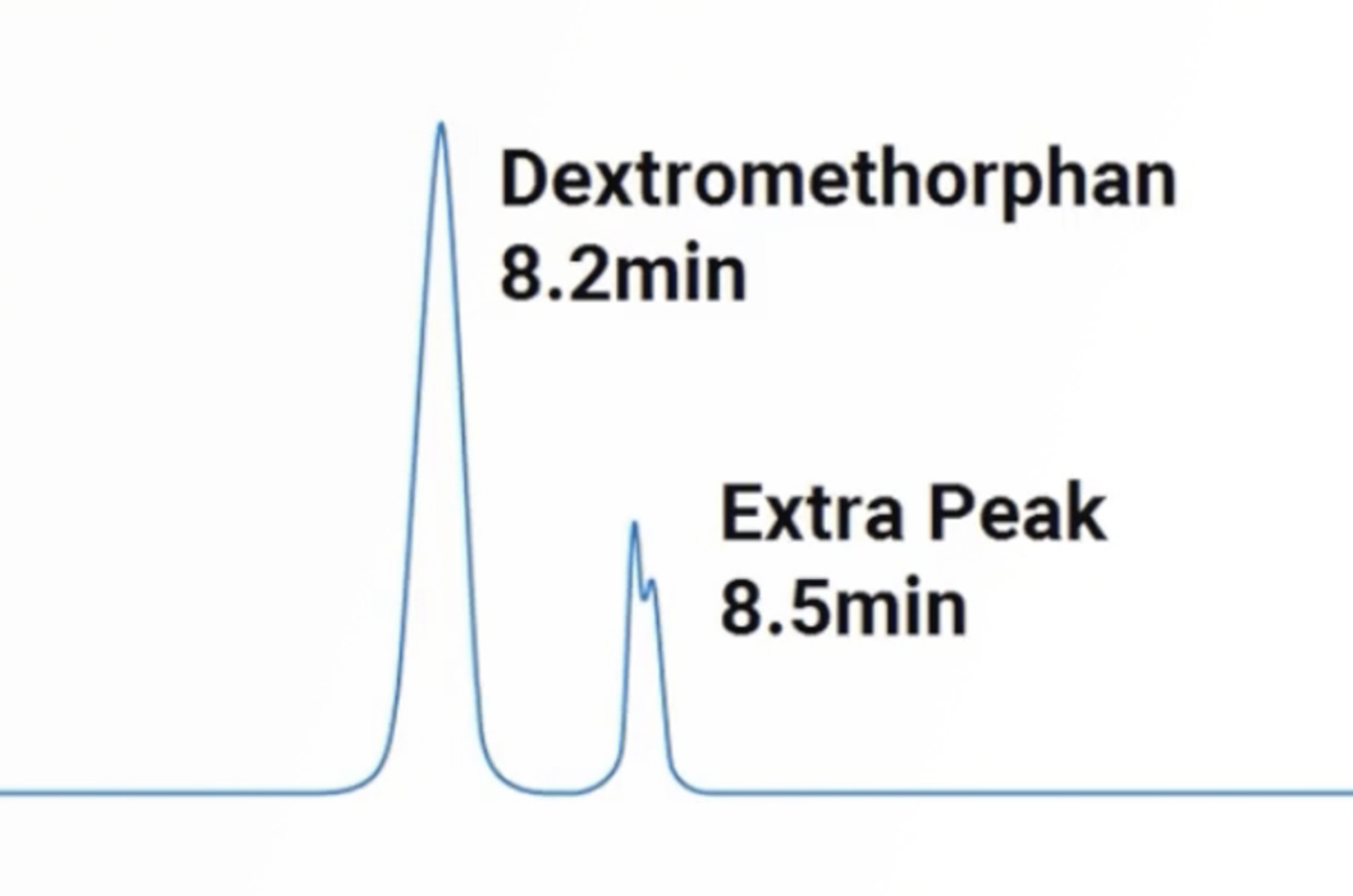
HPLC Extra Peaks and Contamination
The problem of extra peaks and contamination is one of the most common problems the CHROMacademy team get asked about. Therefore, this concise training session will give you quick, practical tips to get you on the path to resolving this complex problem when it arises.
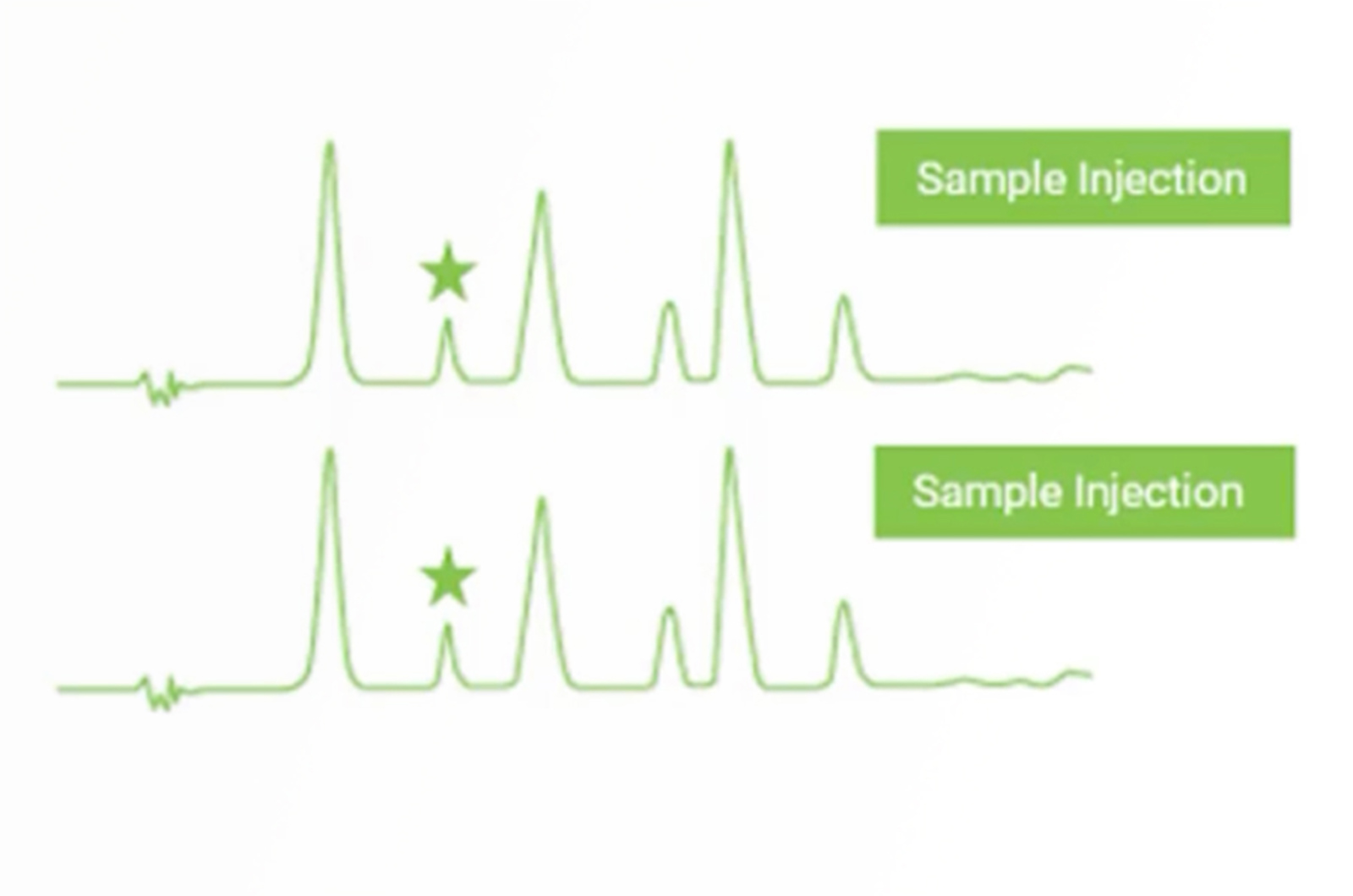
CHROMtalks - Troubleshooting Extra Peaks in HPLC
Let us walk you through a quick troubleshooting strategy for this all-too-common problem of extra peaks in HPLC. In just a few injections we can isolate the problem from a long list of possibilities to just a few, allowing you to quickly focus your troubleshooting efforts. For extra peaks coming from contamination, we will also discuss sources in the mobile phase, sample, HPLC hardware, and column to allow you to build in procedures to help avoid contamination and the occurrence of extra peaks.
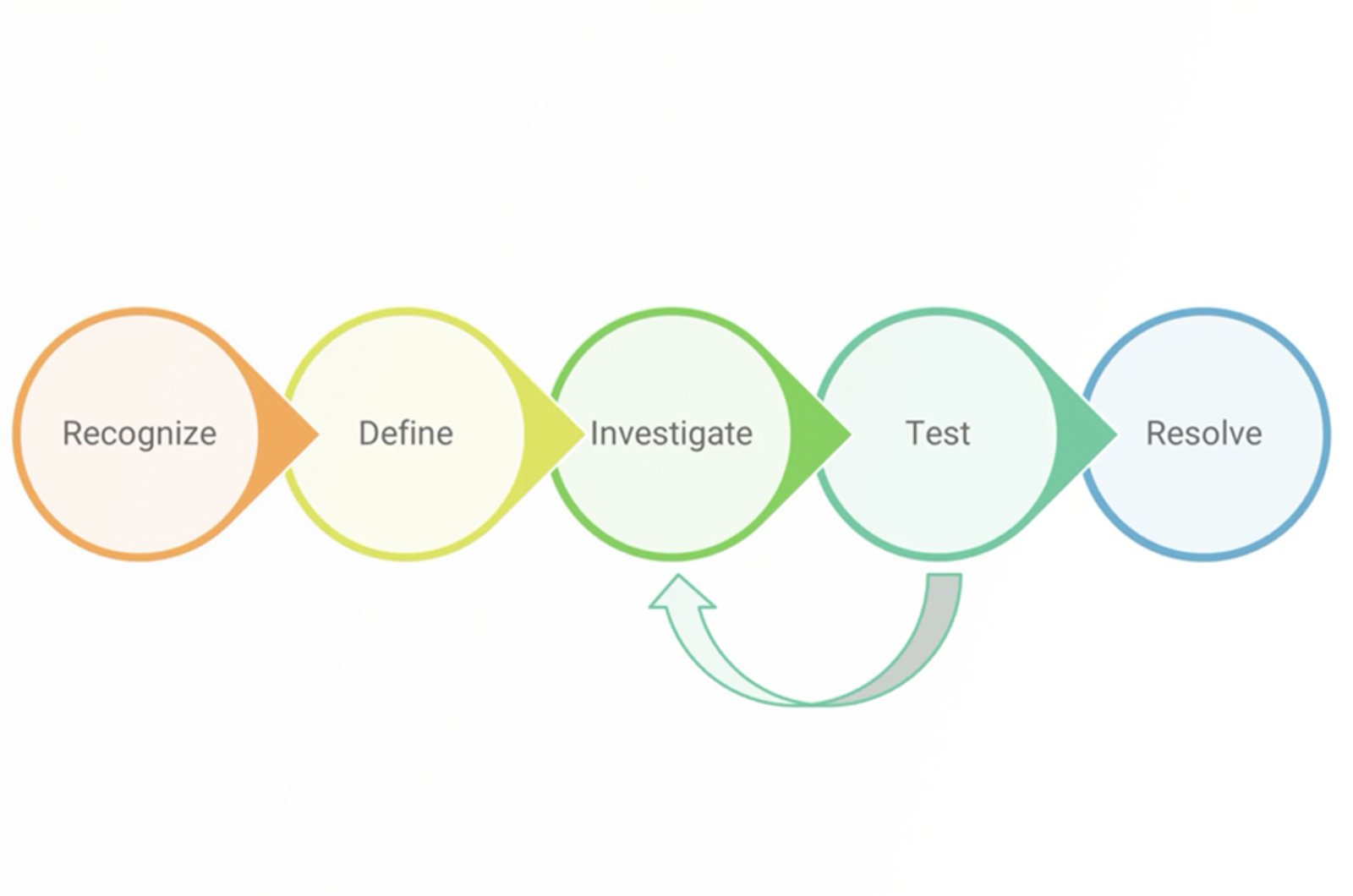
How to Troubleshoot HPLC Extra Peaks and Contamination Like a Pro
Extra peaks resulting from contamination is our most asked about problem. Contamination can be introduced into the HPLC system at any point of the process, making it an incredibly complex and time-consuming issue to troubleshoot. Previously we have discussed the types and sources of contamination. Now it’s time for us to walk you through a strategy to troubleshoot this problem quickly and effectively using simple experiments to narrow down the source of the problem.
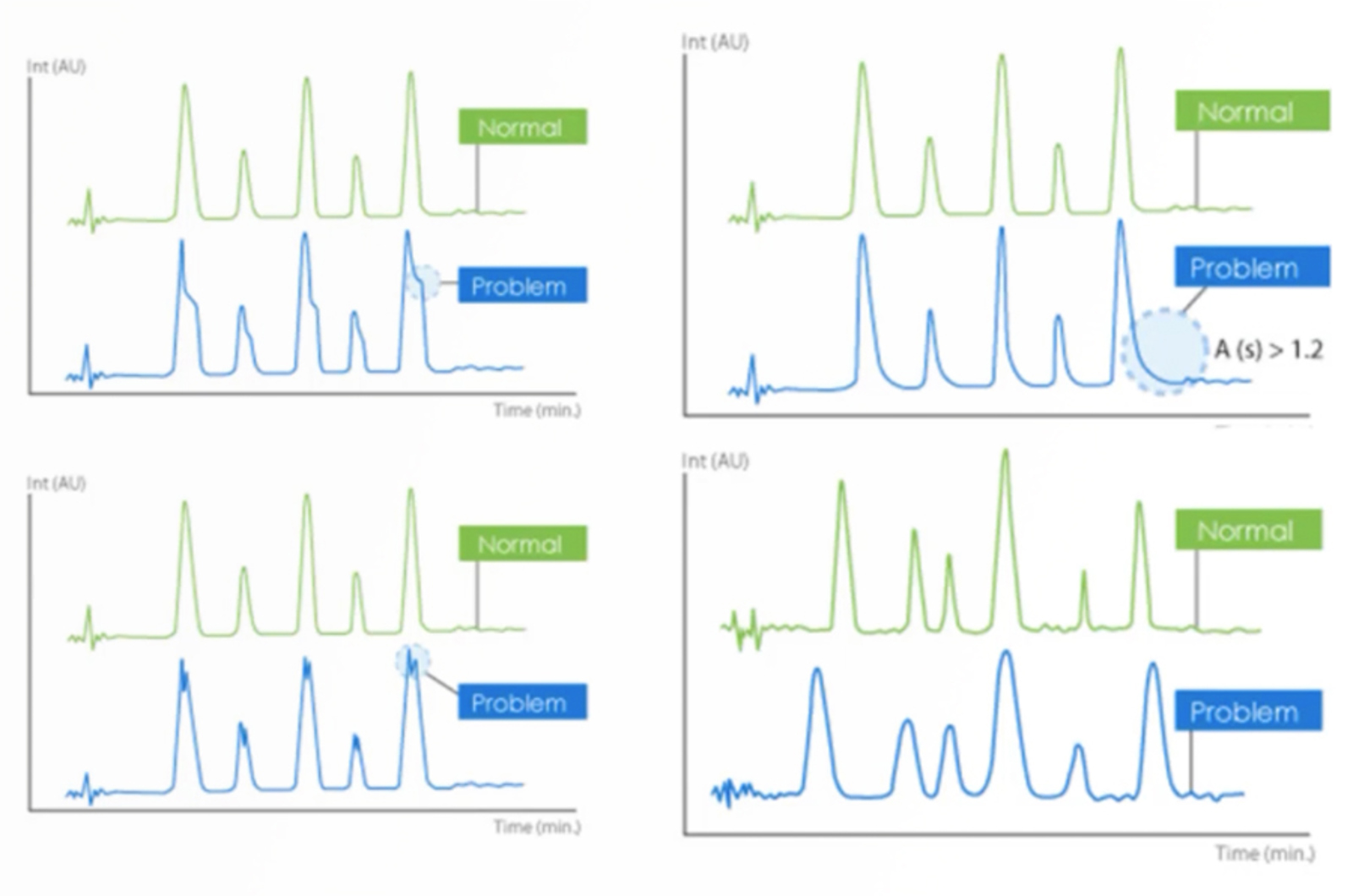
How to Troubleshoot HPLC Extra Peaks and Contamination Like a Pro Q&A
We provided you with a troubleshooting strategy and investigational tools in our webcast - but this is a complex problem to troubleshoot which led to lots of questions being asked. Take an hour and learn even more about troubleshooting extra peaks as we answer your questions, providing you with additional troubleshooting strategies as well as tips and tricks gained from our own experience.
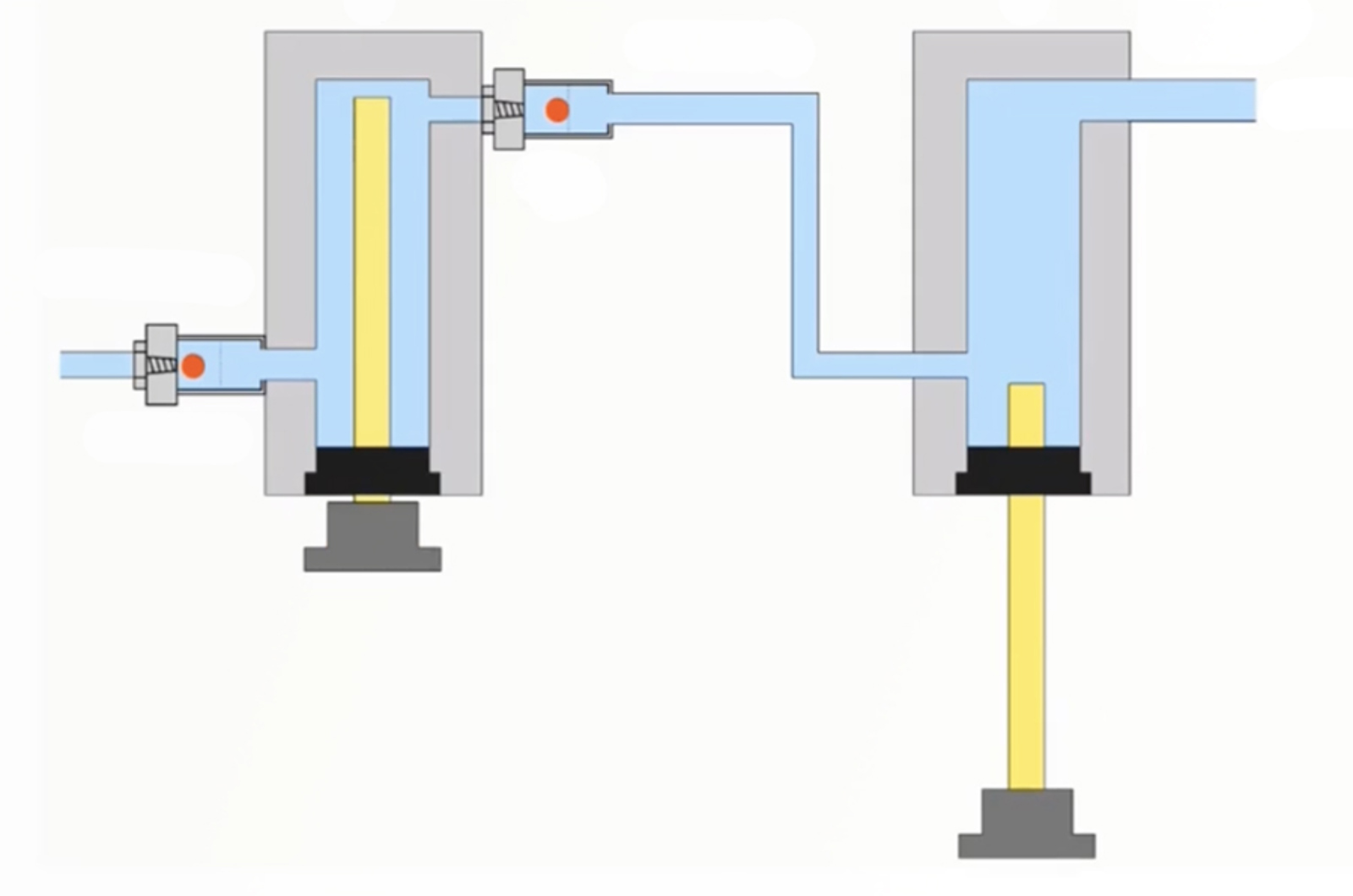
Troubleshooting Pump Issues
This webcast will provide understanding on how HPLC pumps function. This knowledge will allow you to efficiently troubleshoot problems that arise, as well as avoiding them through proper use and maintenance. A focus on common pump problems will allow you to quickly recognize when you have a problem and narrow down the source to ensure instrument down time is kept to a minimum.

Troubleshooting Autosampler Issues
Mechanically complicated, autosamplers have the ability to create a variety of troubleshooting problems. By focusing on each part of the autosampler we will provide you with knowledge of where problems can arise - and how to reduce the likliehood of this through proper use and maintenance. We highlight the common autosampler related problems to ensure that you recognize them and their source quickly to reduce instrument down time.
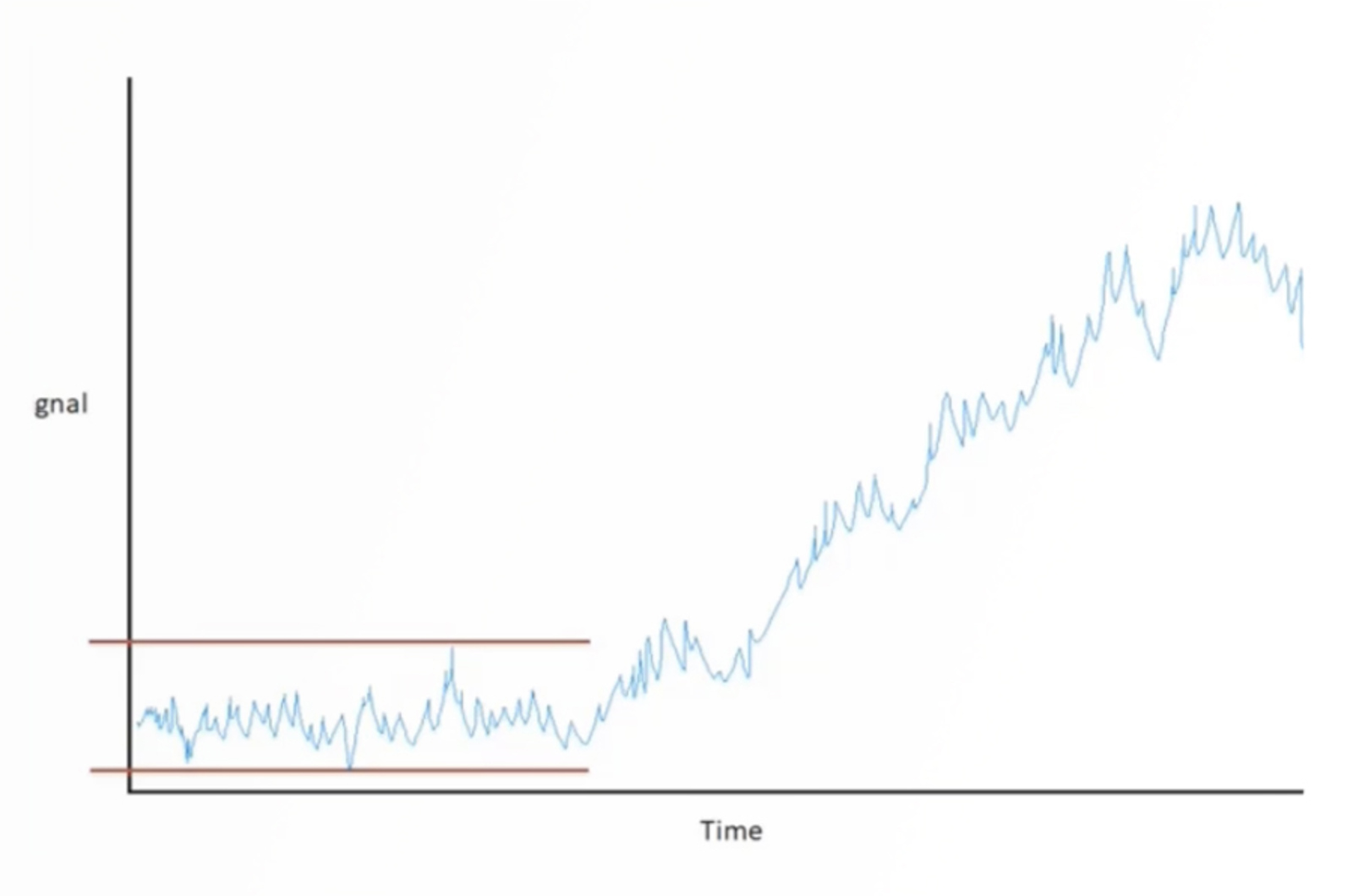
Troubleshooting Sensitivity Issues (HPLC-UV)
This webcast discusses the impact of sensitivity on HPLC-UV methods. We will discuss what sensitivity is, how we measure it, how it is achieved, and some of the problems that result in issues with sensitivity.
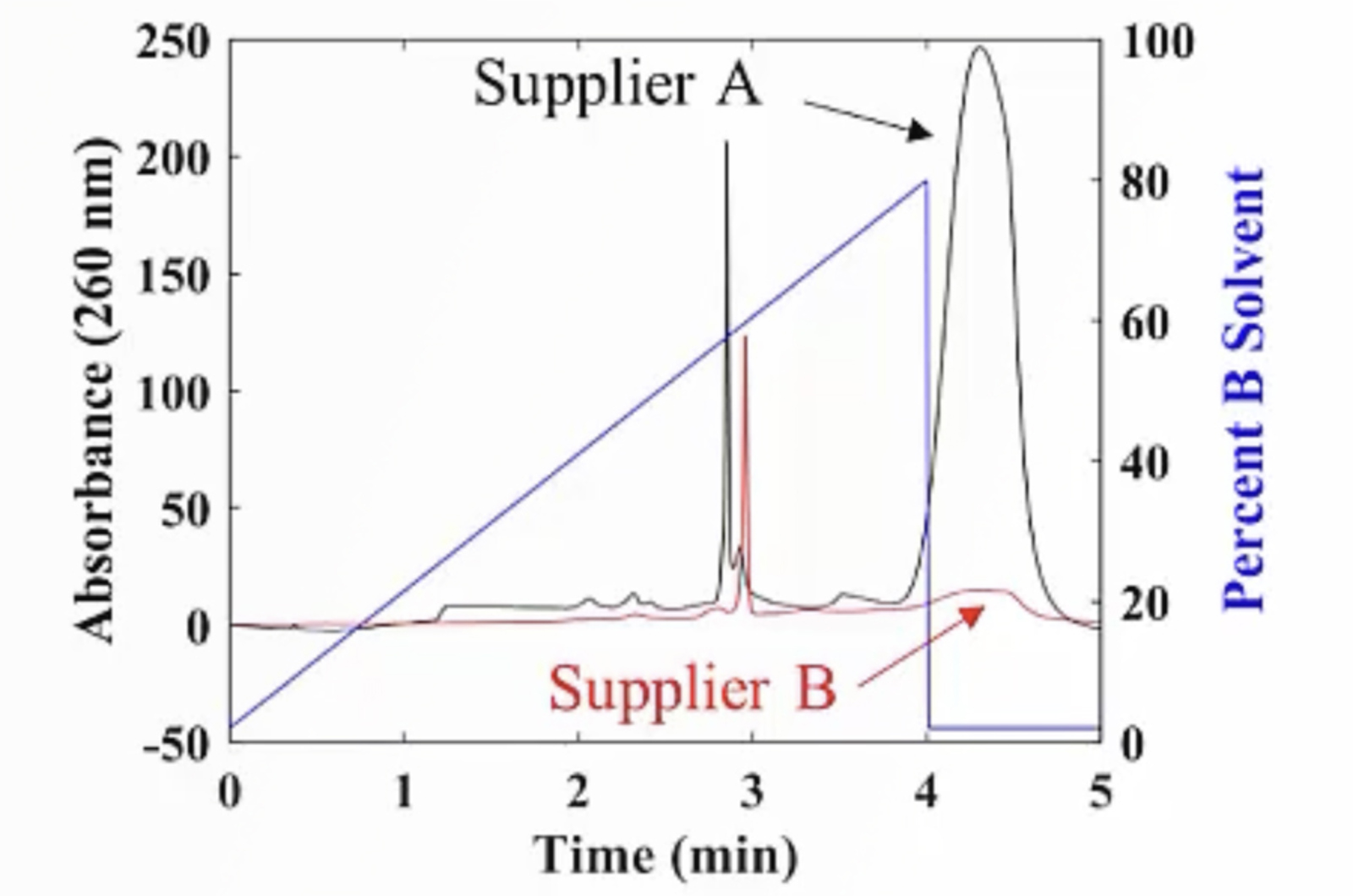
CHROMtalks - Essentials of LC Troubleshooting - Some Problems Just Never Go Away
This webcast will touch on some of the most common troubleshooting problems in HPLC, including best practices for effective troubleshooting, pressure problems, peak shape and width problems, and baseline problems. For each topic we discuss fundamental principles that underpin our troubleshooting approach and use experimental data to illustrate the problems and solutions where possible.

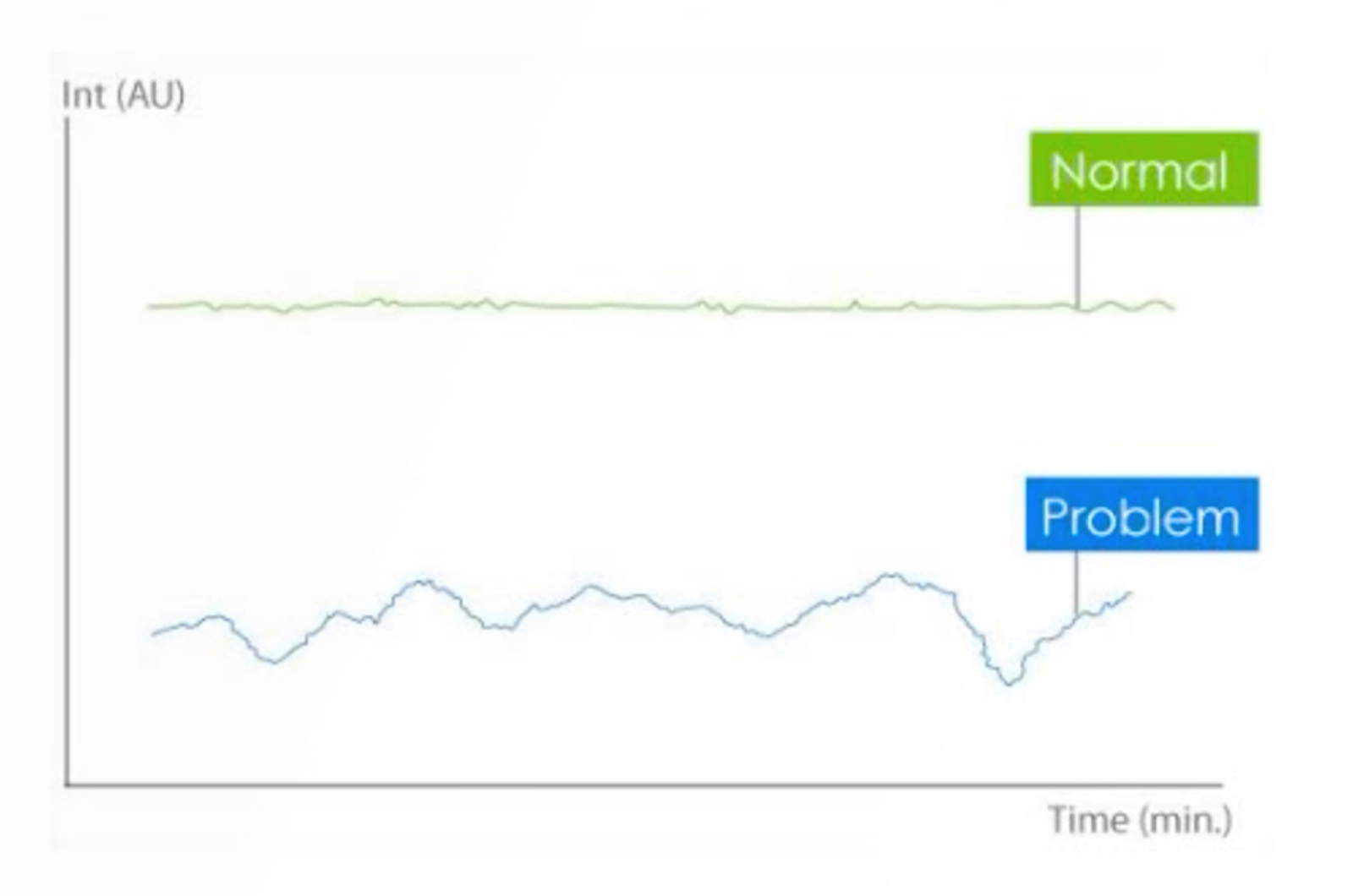
CHROMtalks - Listen to Your LC Instrument - It Is Trying to Tell You Something!
Your LC instrument is always talking. Are you listening? Modern instruments are producing several streams of data and successful users have learned to use that information to their advantage. Troubleshooting a problem becomes much easier when you know how to use this information. This presentation will focus on two of those information streams—pressure and retention time.

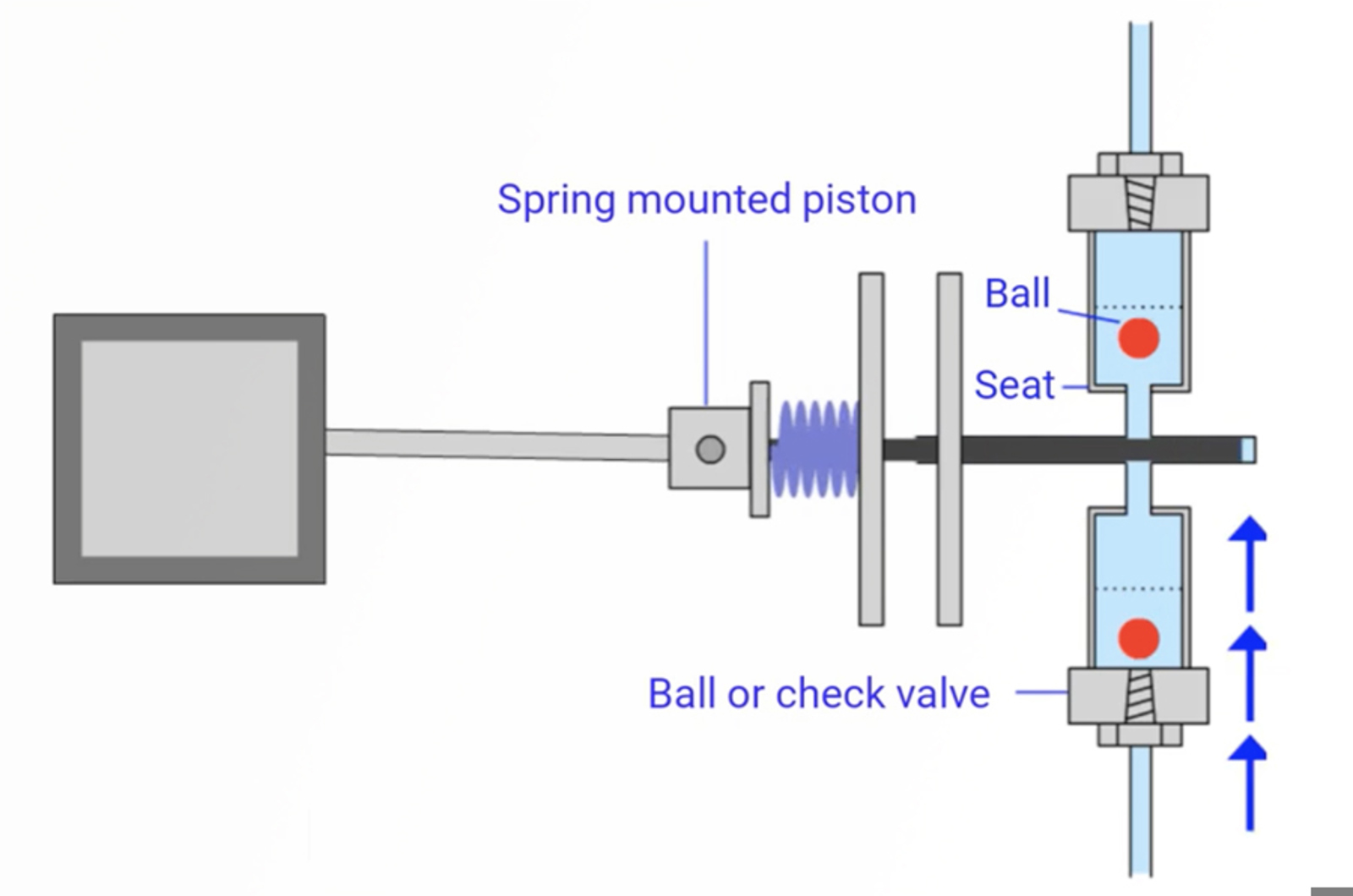
CHROMtalks - The Top Seven Autosampler Problems and How to Avoid Them
This presentation will detail the top seven autosampler issues you are likely to encounter. We’ll explain how to spot these problems, how to fix them, and how to prevent them in the first place, so that you can avoid having to troubleshoot. Topics addressed will include autosampler designs, integral loop autosamplers design and operation, injection valve anatomy, mechanical autosampler issues, and troubleshooting and avoiding carryover.

Troubleshooting HPLC Mobile Phase Issues - Q&A
The HPLC mobile phase impacts retention and selectivity of analytes, therefore, mistakes in designing and preparing mobile phases will lead to considerable problems. Our webcast looking at the mobile phase parameters which are most likely to cause problems generated a lot of questions. So we've answered them. If someone is asking the question, the answer is worth knowing.

HPLC Column Troubleshooting - Can We Fix It?
This webcast will provide useful practical advice on troubleshooting issues relating to HPLC columns; and how to minimize and resolve these.
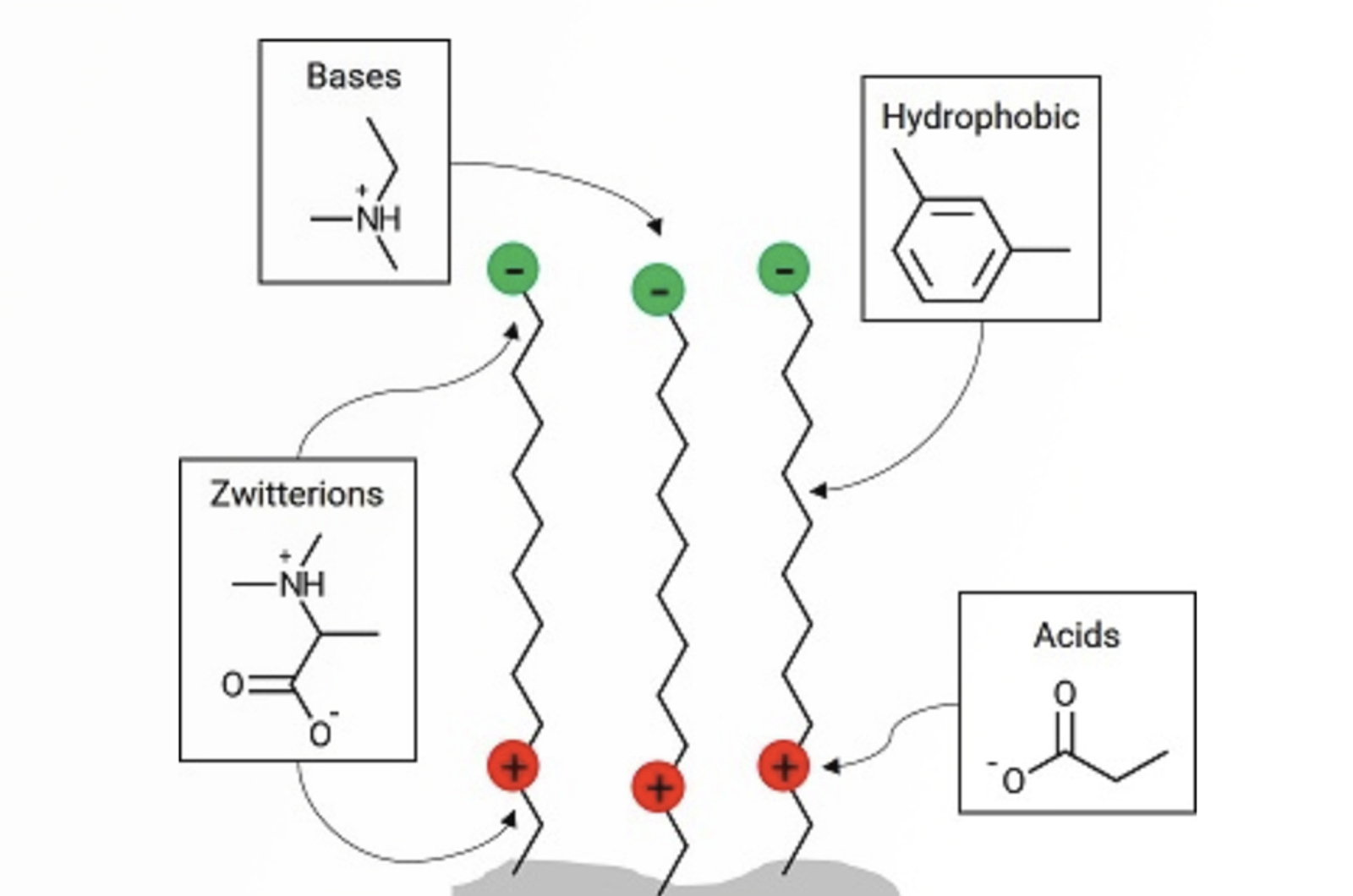
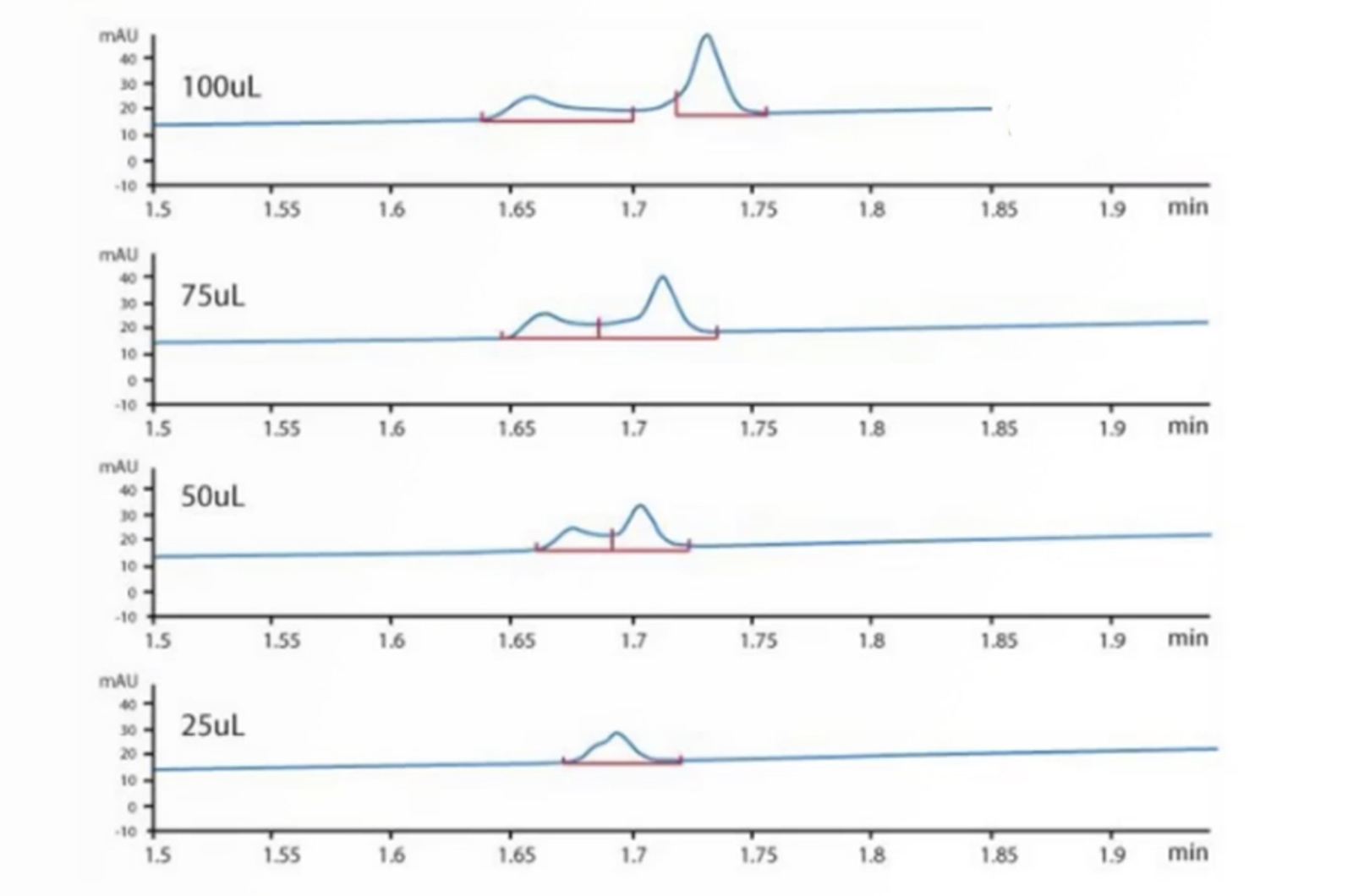
Avoiding Problems Associated With HPLC Column Overload
Much has been written about column overload, however, we have seen many instances in which overload may not be instantly recognized, or causes problems that may not normally be attributed to overload. Therefore, we want to explain in this quick guide how to spot and deal with different types of overload situations.

CHROMtalks - LC Troubleshooting: Back To Basics
Through real-world examples and practical solutions, this talk aims to demystify LC troubleshooting and help new users feel confident to optimize their systems, keep their instruments running smoothly and avoid costly lab downtime.

CHROMtalks - Troubleshooting and Optimizing Sample Introduction in HPLC
This talk will explore the impact of HPLC injection variables on peak shape and provide general recommendations for users who are developing methods.

CHROMtalks - HPLC Re-Analysis: Root Cause Analysis
This webcast provides valuable insights to help laboratories improve quality and data integrity of HPLC workflows.

