Fundamentals of SPE Mechanisms
Course Details
Quick Guide
Premier
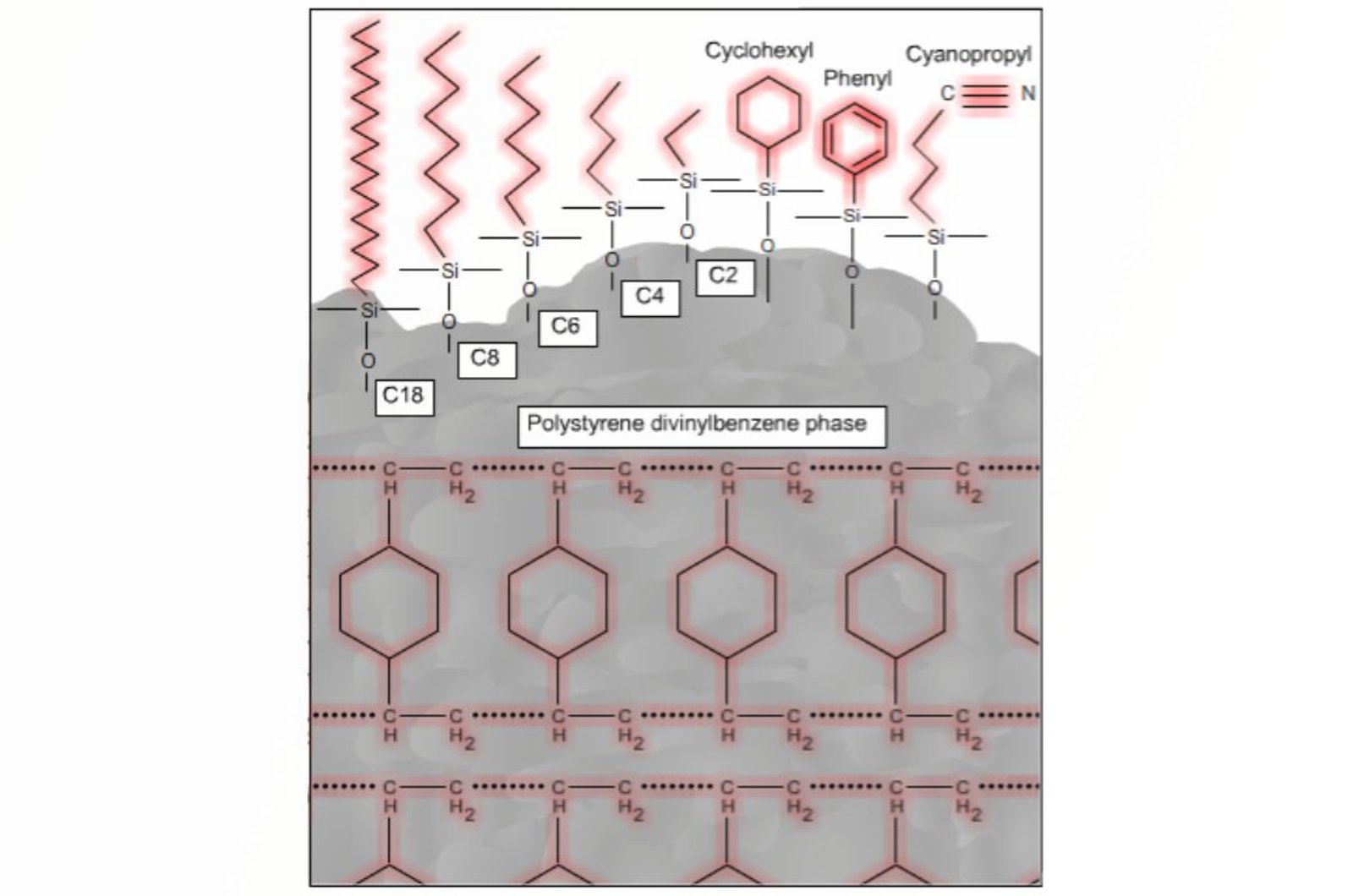
Solid phase extraction (SPE) is a highly selective mode of sample preparation, akin to the familiar principles of column chromatography. The range of currently available SPE sorbent chemistries and new technologies makes this applicable to a wide variety of analytes in various application areas.
In this article we will cover some of the basic scientific principles behind SPE in order to allow the correct mode of extraction to be selected, through an understanding of how analytes interact with and are separated by the sorbent.