I Only Ever Need C18 for Reversed Phase Chromatography Don’t I?
Course Details
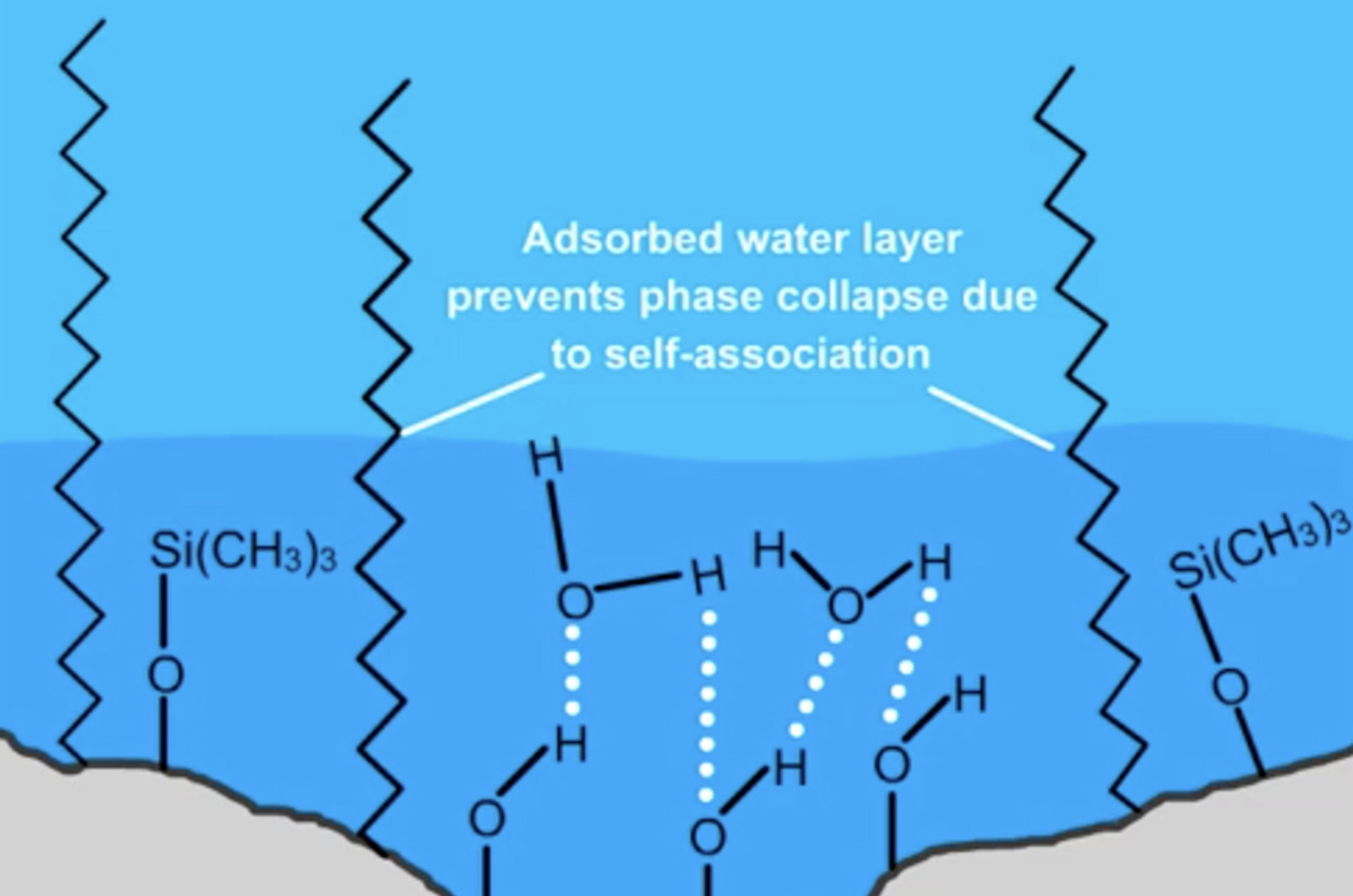
We’re probably all guilty at some point of starting all new method development projects with our favourite and most trusted C18 column installed on the system. Whilst are old favourite C18 may perform an adequate job it may lead to a lot more work during method development or a method that it is non-optimal.