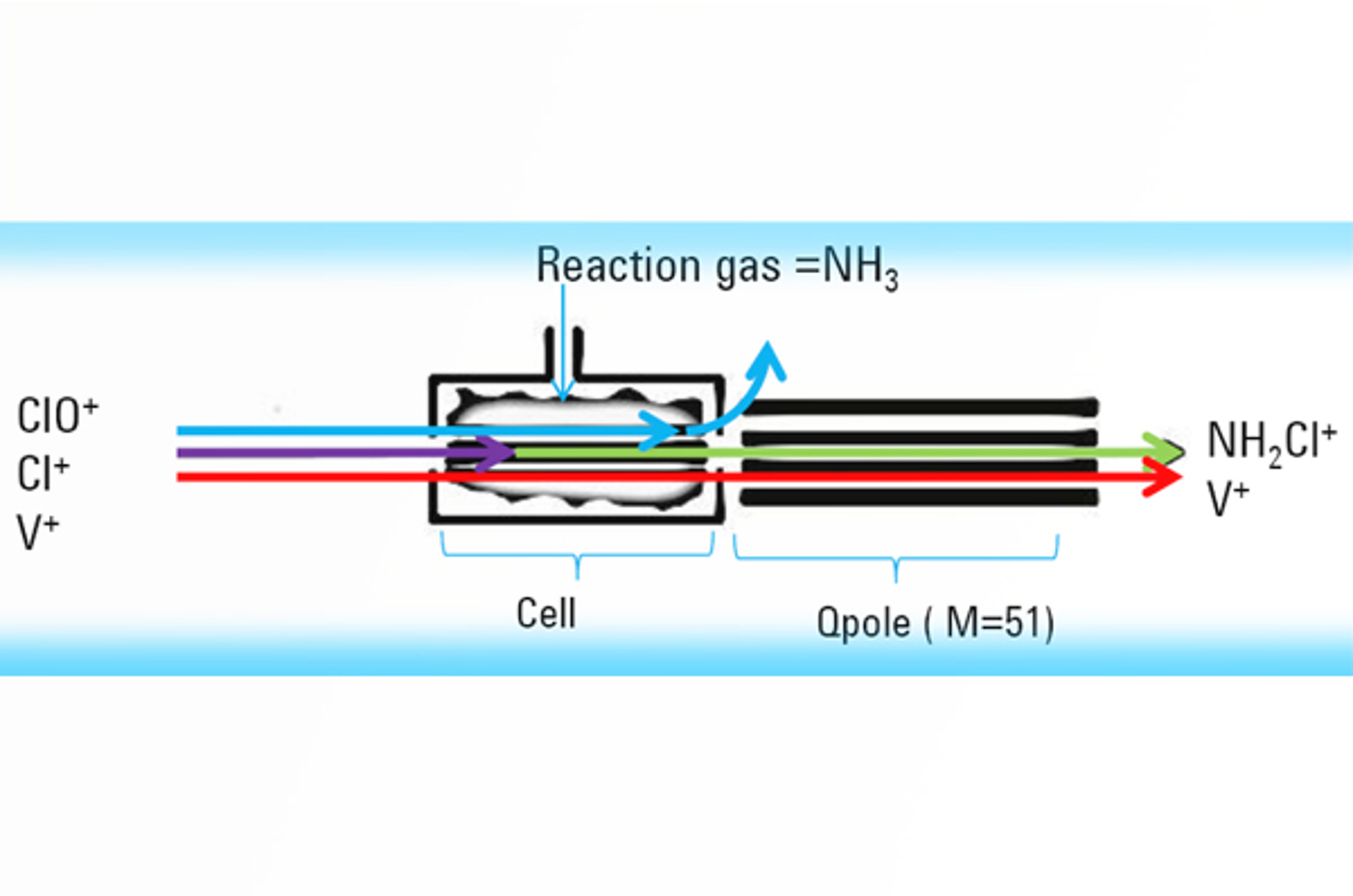
Fundamentals of ICP-MS
Course Details
Webcast
This webcast will provide you with the fundamental theory and knowledge of the instrumentation which will allow you to implement ICP-MS analyses in your laboratory. We will discuss the general instrument setup, how the plasma is formed, and how samples are introduced into the system, as well as looking at some basics of data interpretation.
Topics include:
- What can ICP-MS do?
- General instrument overview
- Plasma formation
- Which gases are used and why?
- Sample introduction
- How to interpret data
- Interferences in ICP-MS