Technique
Explore essential analytical sample preparation techniques to improve accuracy and reproducibility in chromatographic analysis. Learn liquid-liquid extraction (LLE) for separating compounds based on solubility. Understand Soxhlet extraction, ideal for solid-liquid extractions in environmental and food analysis. Discover QuEChERS, a fast and effective method for pesticide residue analysis. Optimize solid phase extraction (SPE) for clean sample enrichment and purification. Enhance sample clarity with filtration techniques, ensuring reliable results in chromatography and mass spectroscopy. Designed for professionals in pharmaceutical, food safety, environmental, and forensic sciences, these courses include multimedia learning, webcasts, quick guides, and practical tips for effective sample processing. Start learning today to refine your sample preparation skills and achieve high-quality analytical results.
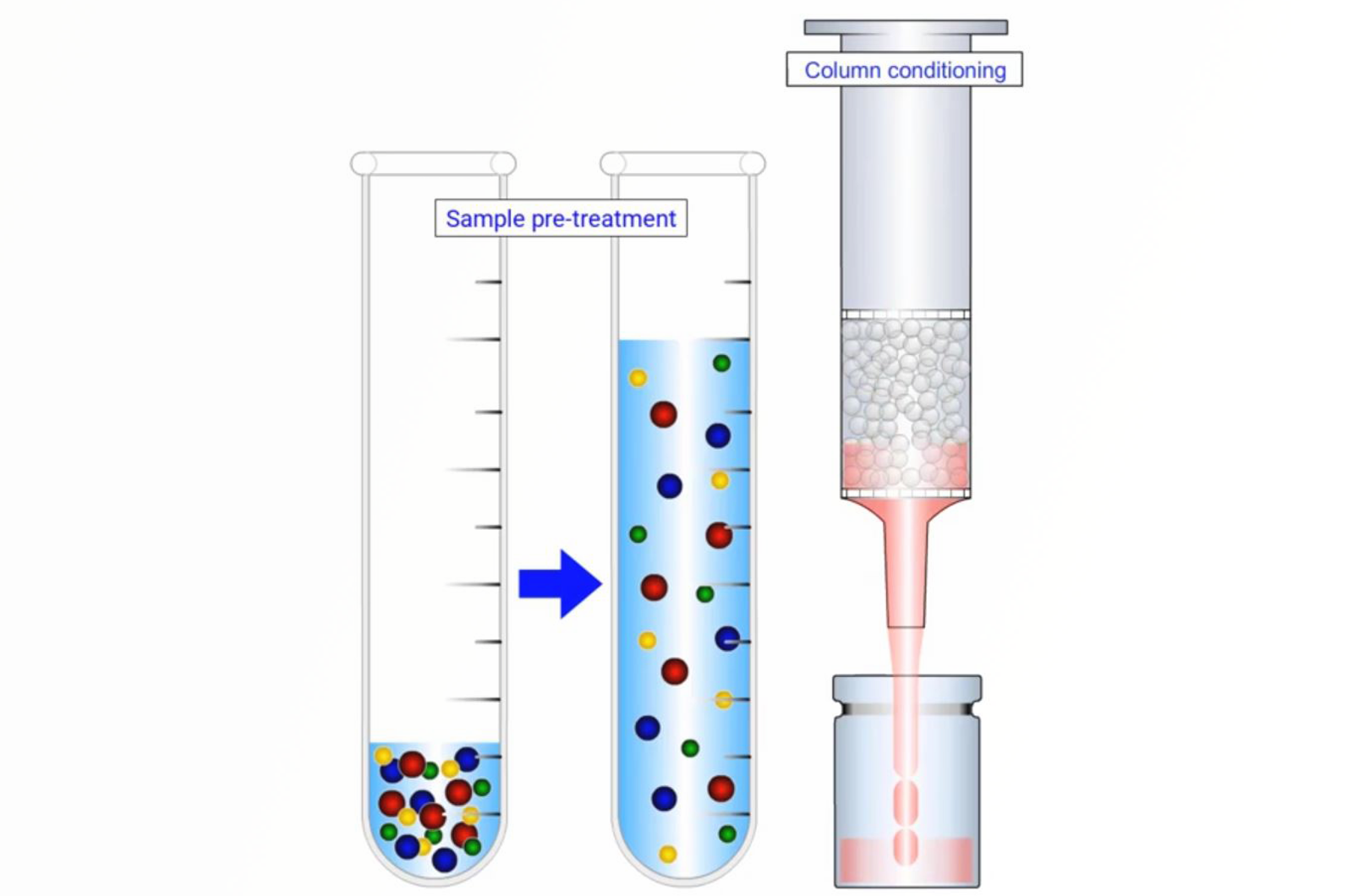
SPE Mechanisms
The purpose of this module is to highlight the various steps in the protocol for various modes (sorbents) for conventional solid phase extraction procedures including: non-polar SPE, polar SPE, cation exchange SPE, anion exchange SPE, and mixed-mode SPE. For each mode the various portocol steps are examined in terms of the solvents and solutions used, sorbents used, solvent additives, pH, and ionic strength manipulation.
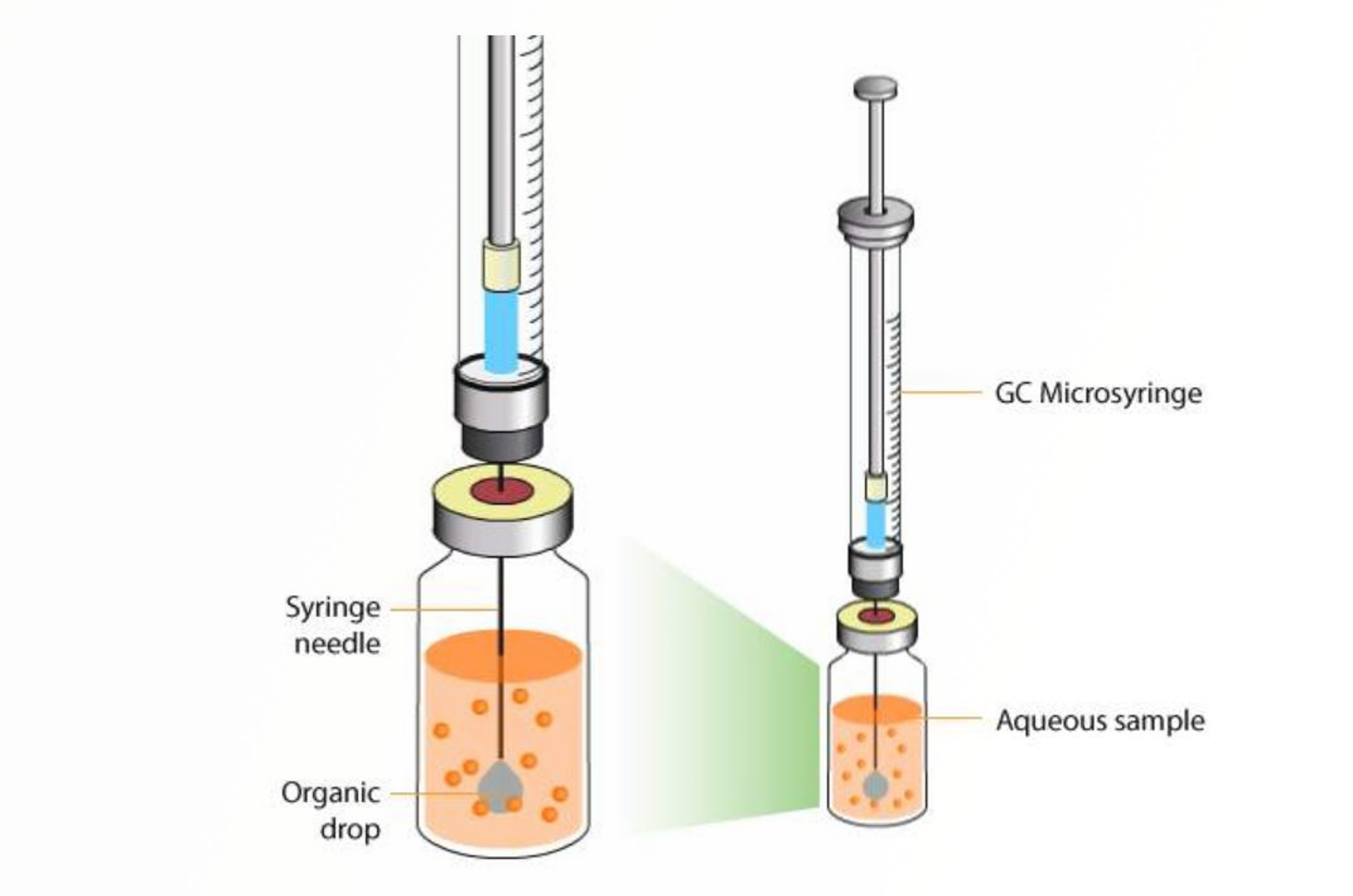
Microextraction Techniques
This module covers the theory of key microextraction techniques, including, single drop microextraction (SDME) and solid phase microextraction (SPME).
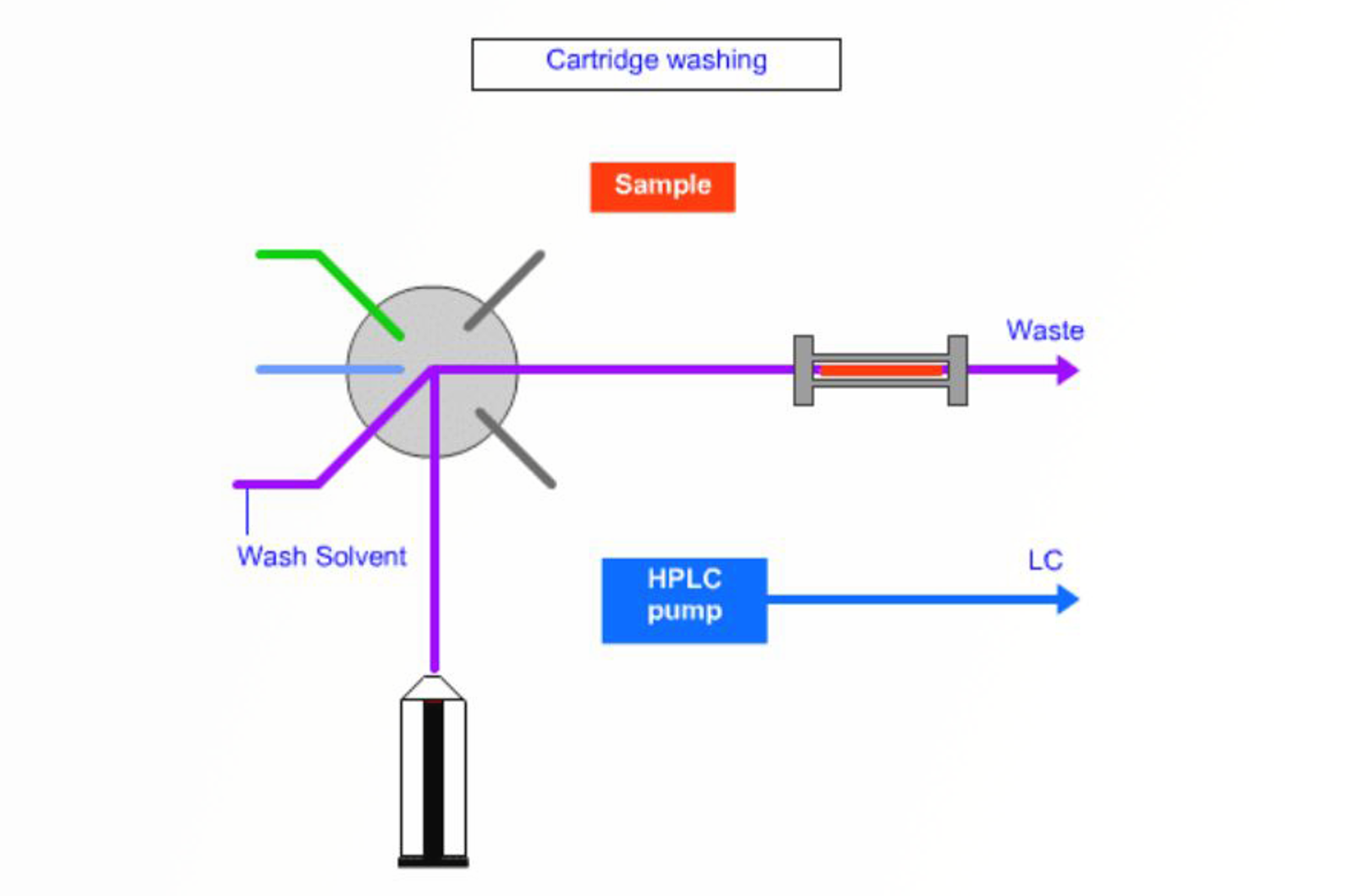
Approaches to Automation for SPE
The aim of this module is to introduce the principles of automation for solid phase extraction, and present fundamental concepts regarding molecular imprinted polymer sorbents.
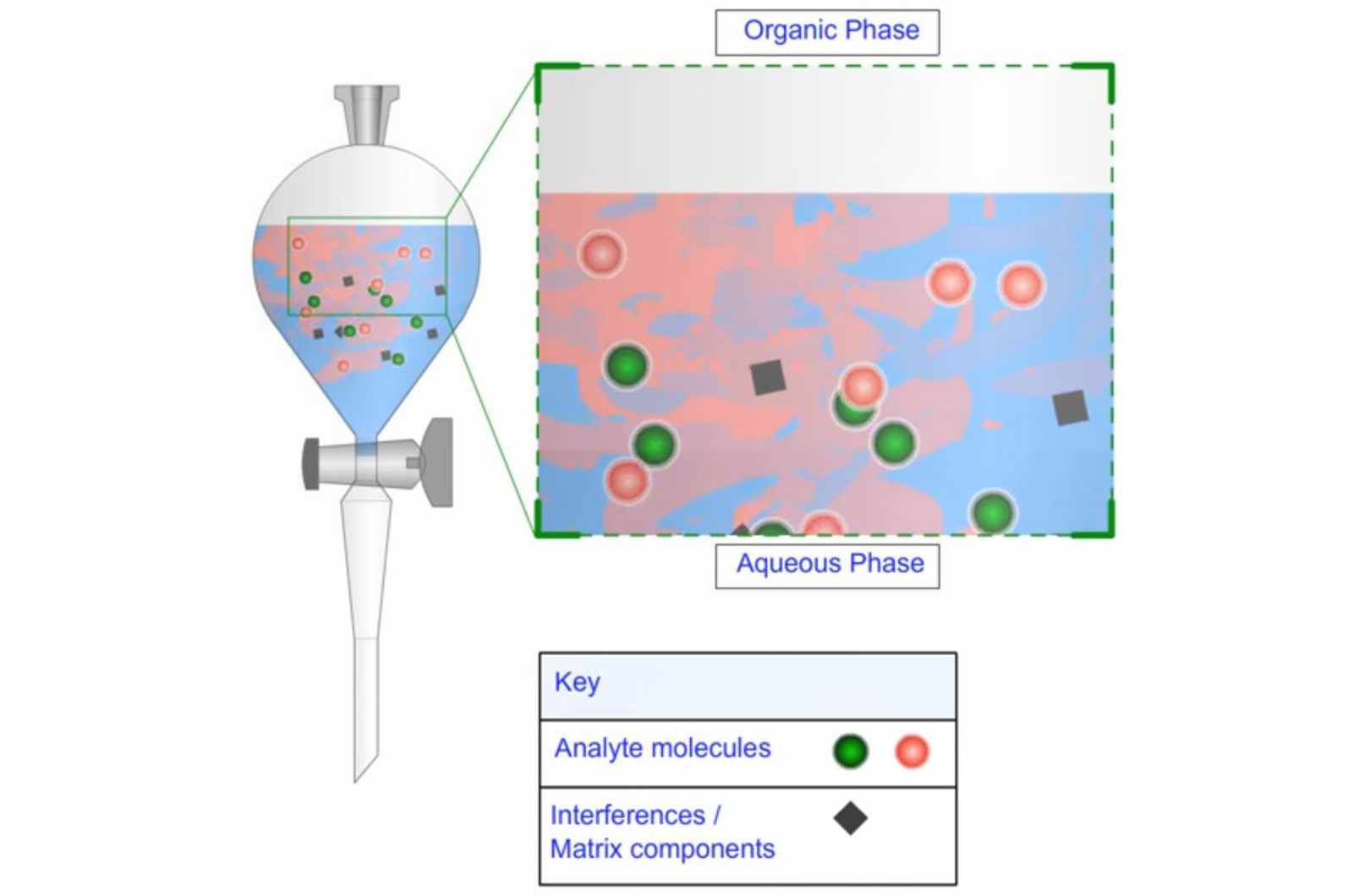
Liquid/Liquid Extraction Techniques
In this module, the basic principles of liquid/liquid extraction (LLE) are described, as well as an overview of limitations and drawbacks of LLE protocols. Specific advice is given for various problems that are routinely encountered. Emulsions and how to deal with them is discussed, and support-assisted liquid/liquid extraction (SALLE) is presented as a useful variation on standard liquid/liquid extraction.
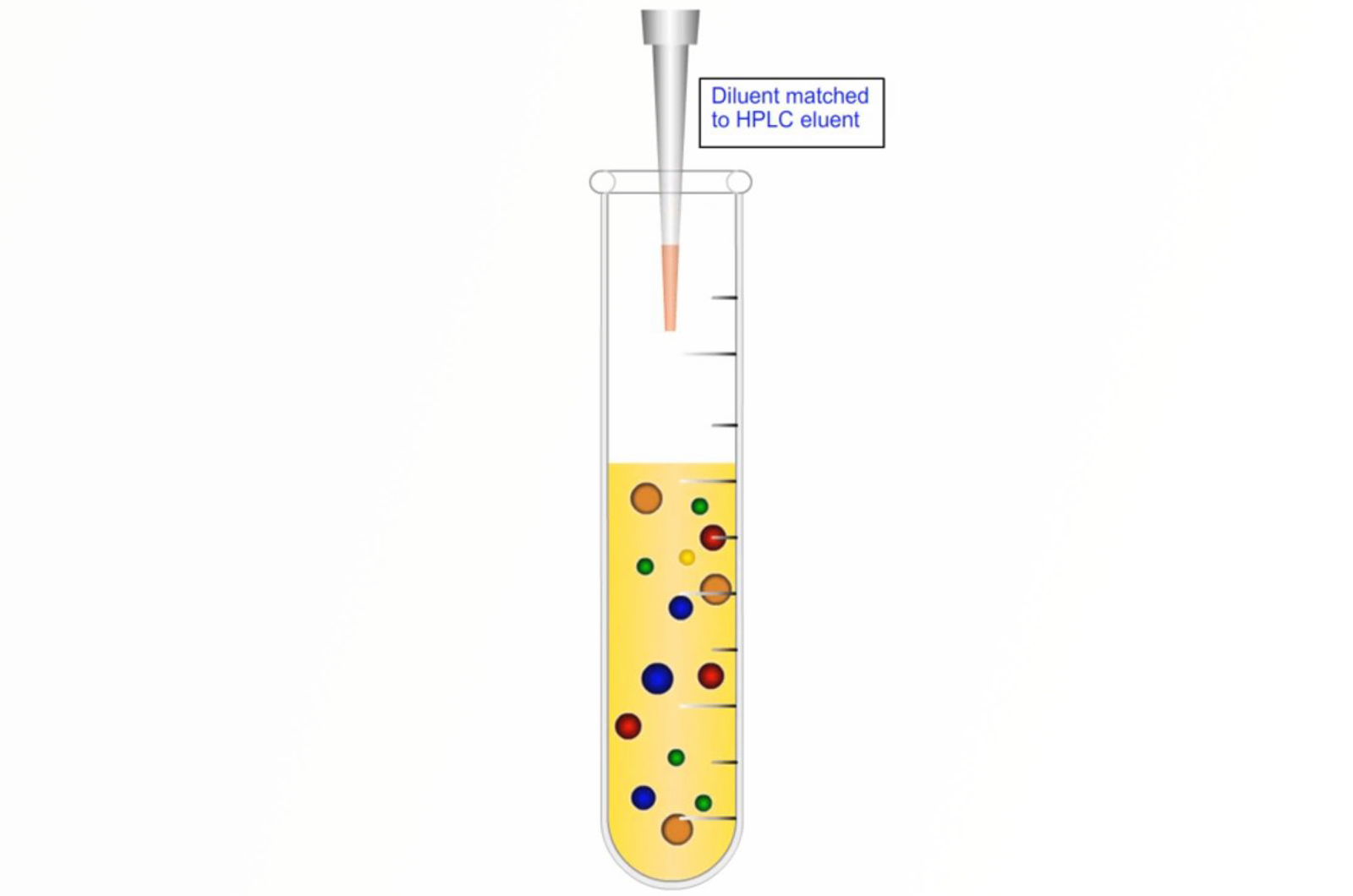
Primary Sample Preparation Techniques
This module takes an initial look at the science behind the most commonly used sample preparation techniques.
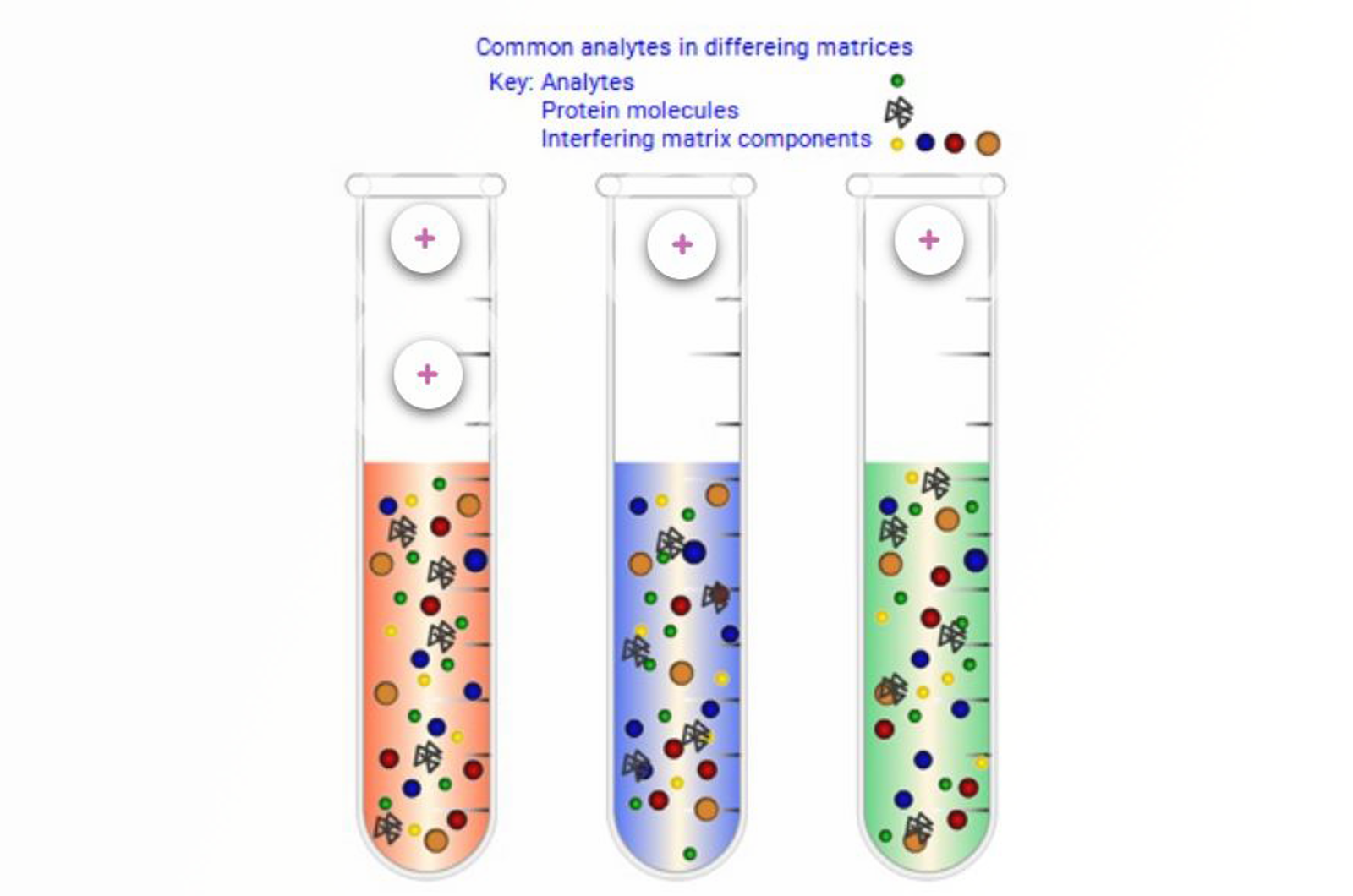
Molecular Properties
This module introduces the concept of functional group chemistry as a means of targeting separation mechanisms in liquid and solid phase extraction (SPE).
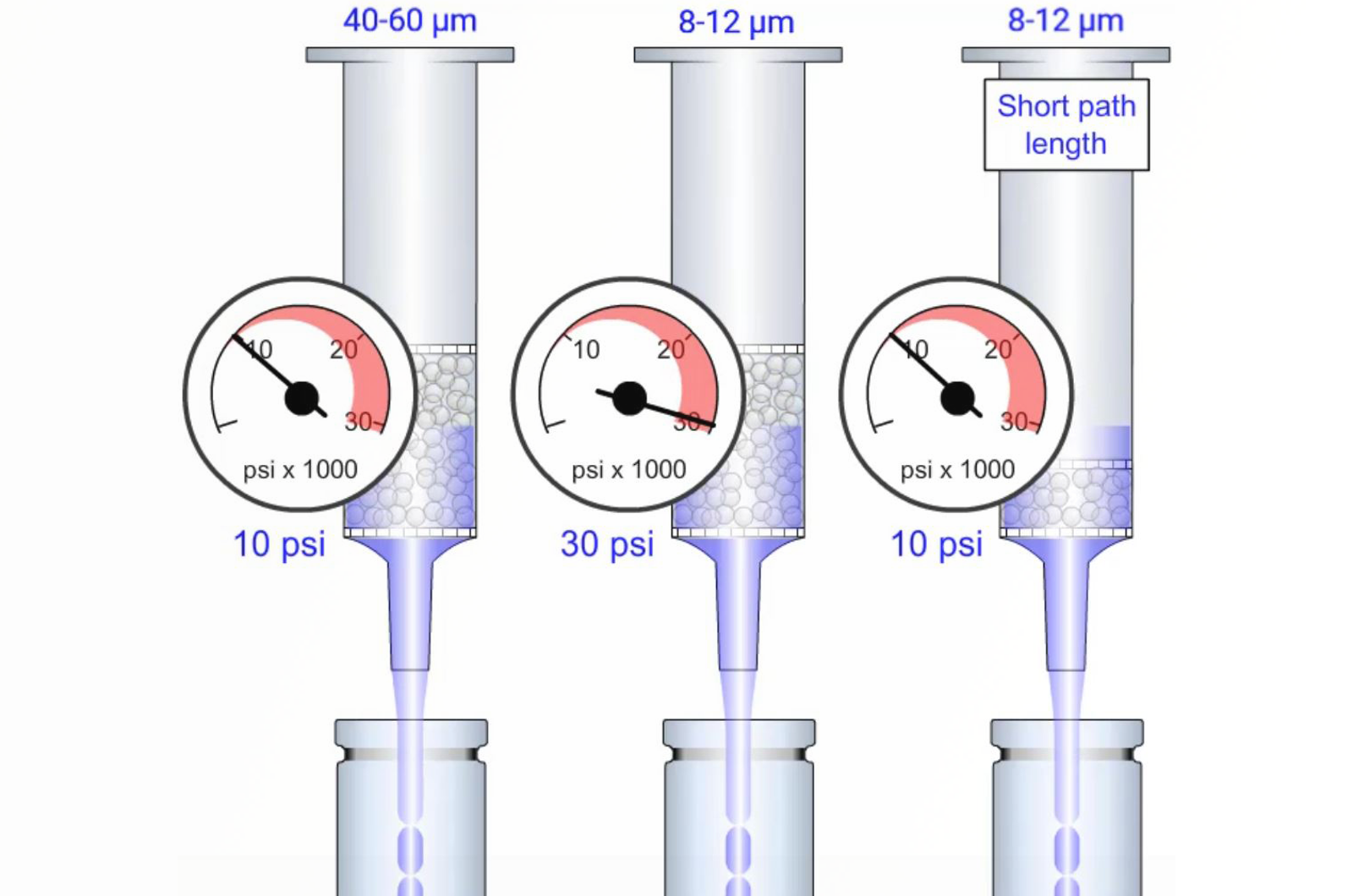
SPE Overview
This module introduces the terminology associated with solid phase extraction (SPE). It explains the chemistry and physical properties associated with SPE sorbents and substrates, the different steps in a solid phase extraction protocol, sorbent selection, eluent solvent strength optimization, and the use of pH and ionic additives to control selectivity in SPE.
Choosing the Correct Sample Preparation Technique
This webcast will provide details of sample preparation techniques and guide you towards the correct method for your sample; whether it is a simple liquid/liquid extraction or a more complex solid phase extraction. It is explained what sample preparation techniques are available, and how to choose the correct method.
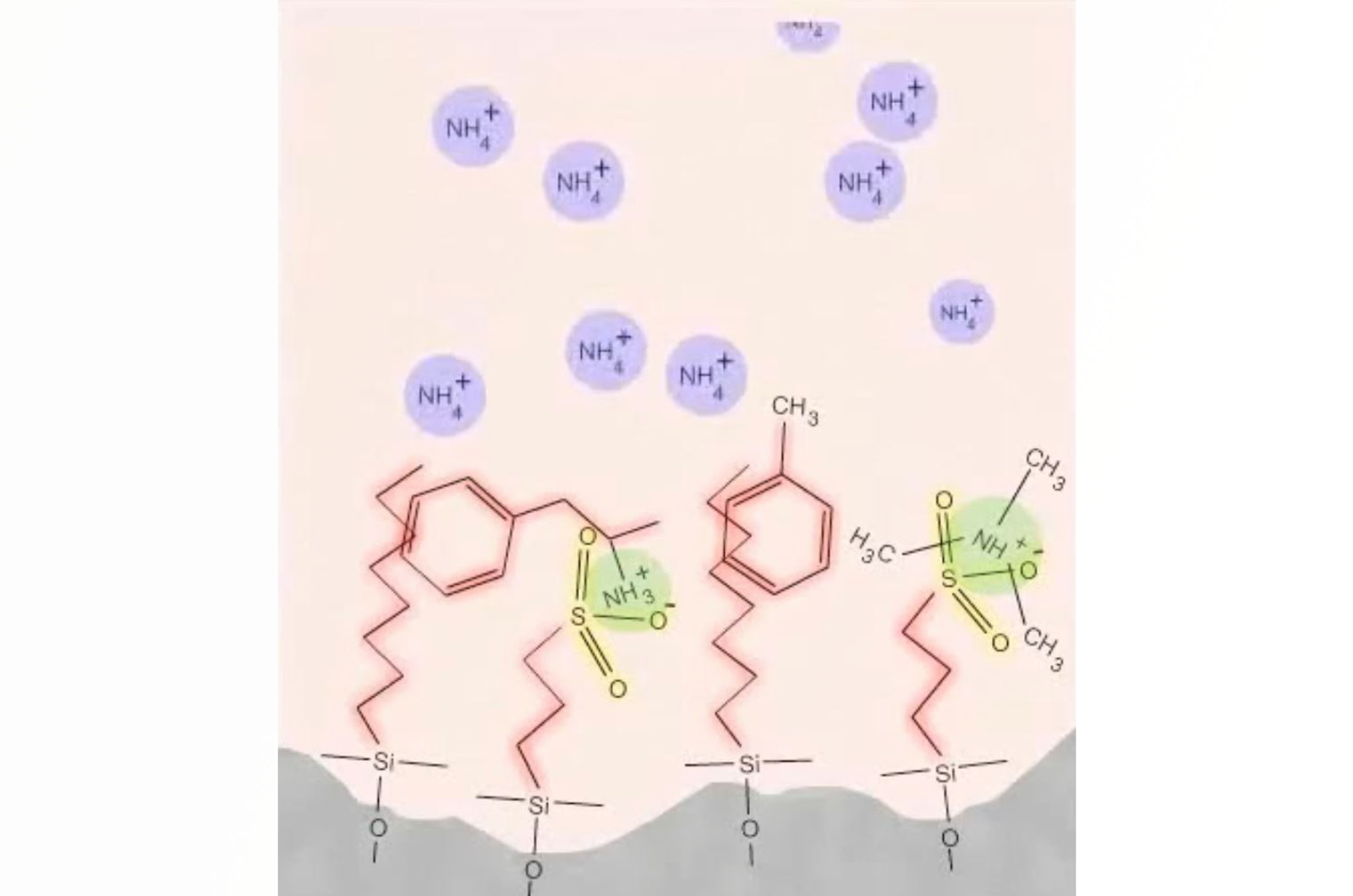
Understanding the Mechanisms of Solid Phase Extraction
This webcast is aimed to help you to understand all of the key stages in SPE protocol development. This understanding is crucial for improving your SPE technique and ultimately to developing better solid phase extraction methods.
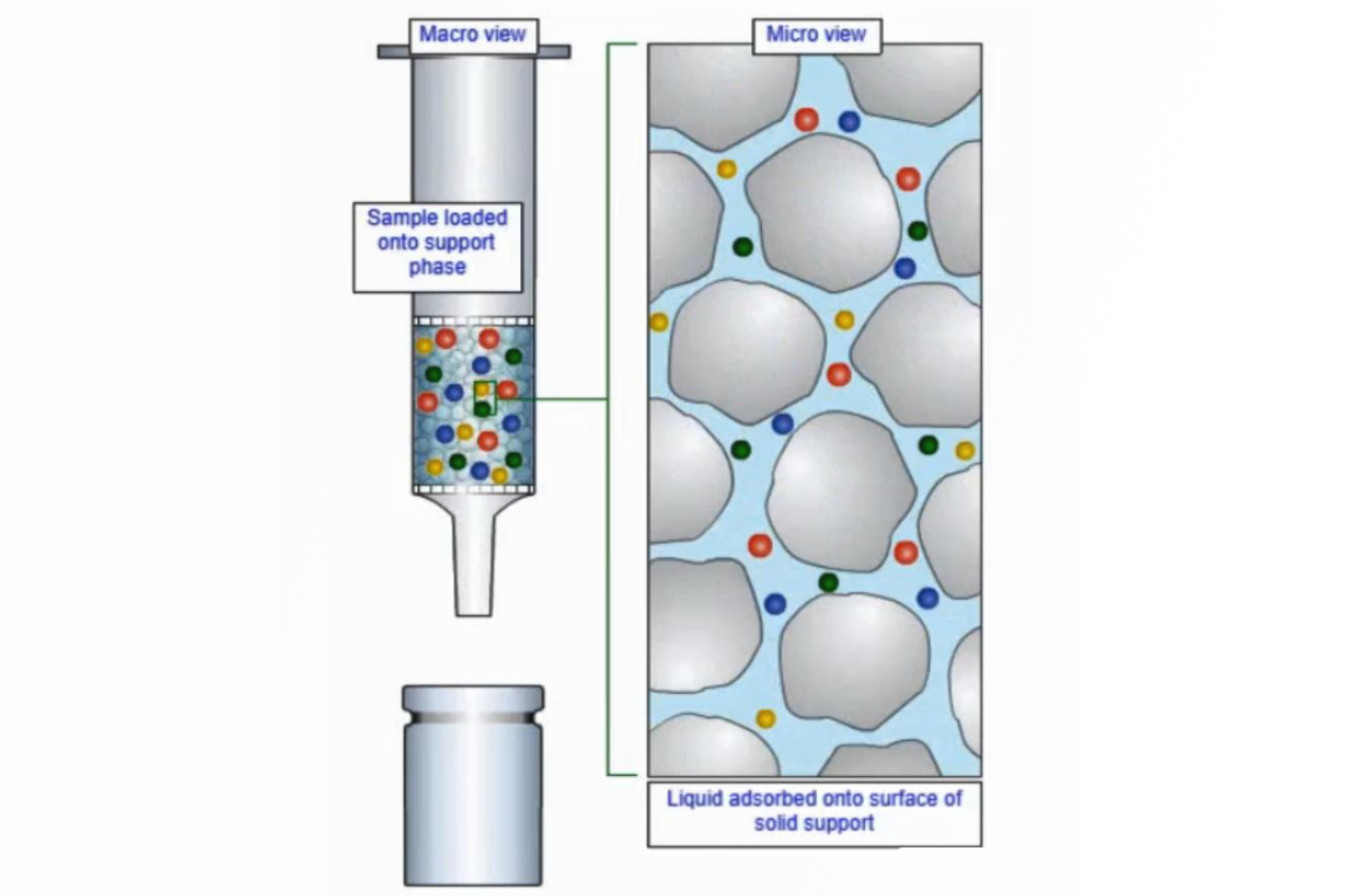
Sample Preparation - Supported Liquid Extraction (SLE)
This quick guide introduces supported liquid extraction (SLE), a variation of liquid/liquid extraction (LLE).
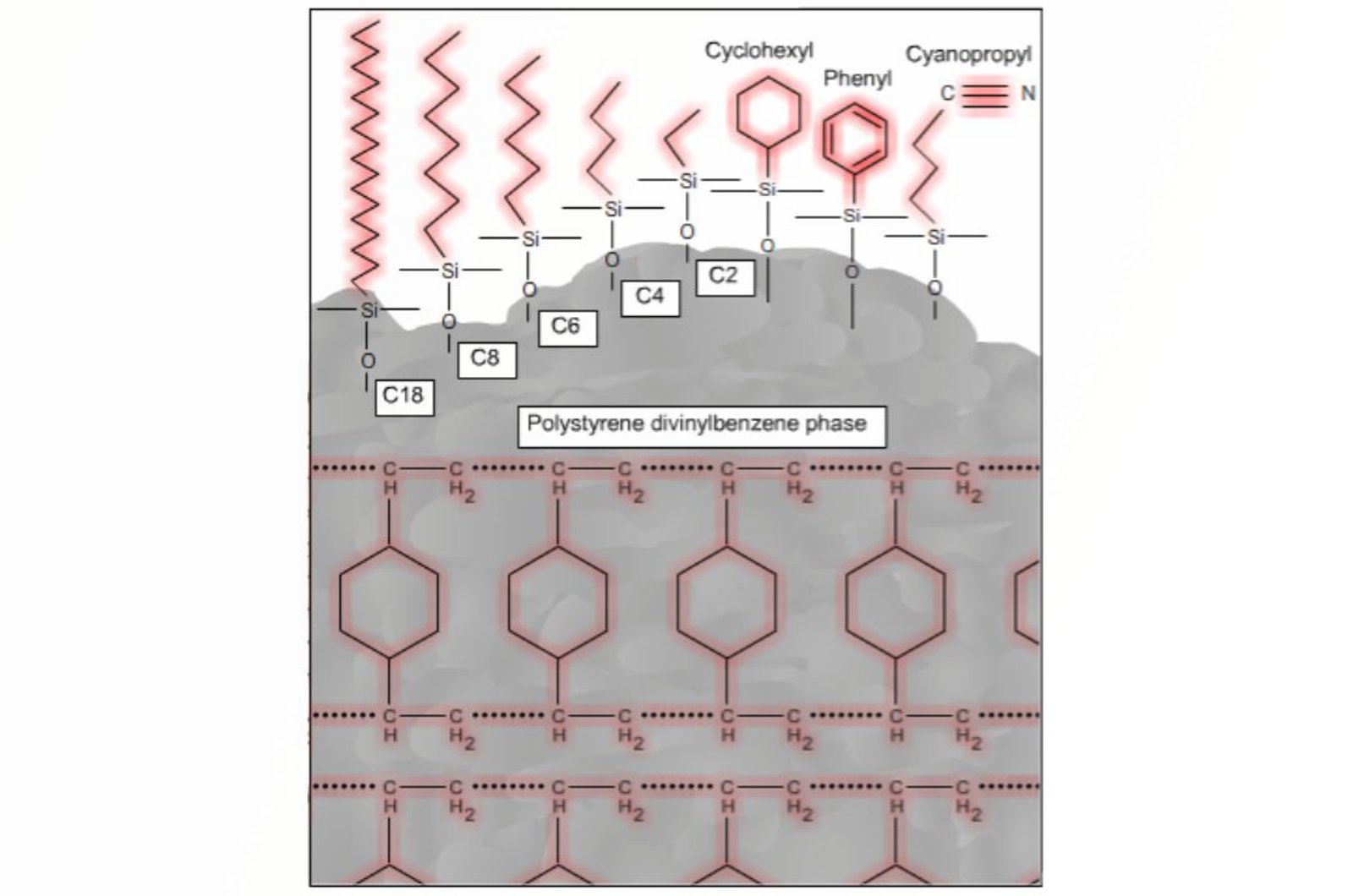
Fundamentals of SPE Mechanisms
In this article we will cover some of the basic scientific principles behind SPE in order to allow the correct mode of extraction to be selected, through an understanding of how analytes interact with and are separated by the sorbent.

SPE - What Can You Learn in 5 Minutes?
This quick guide focusses on the three main parameters to optimize in SPE, which are the choice of column sorbent, the choice of wash solvent, and the choice of eluting solvent.
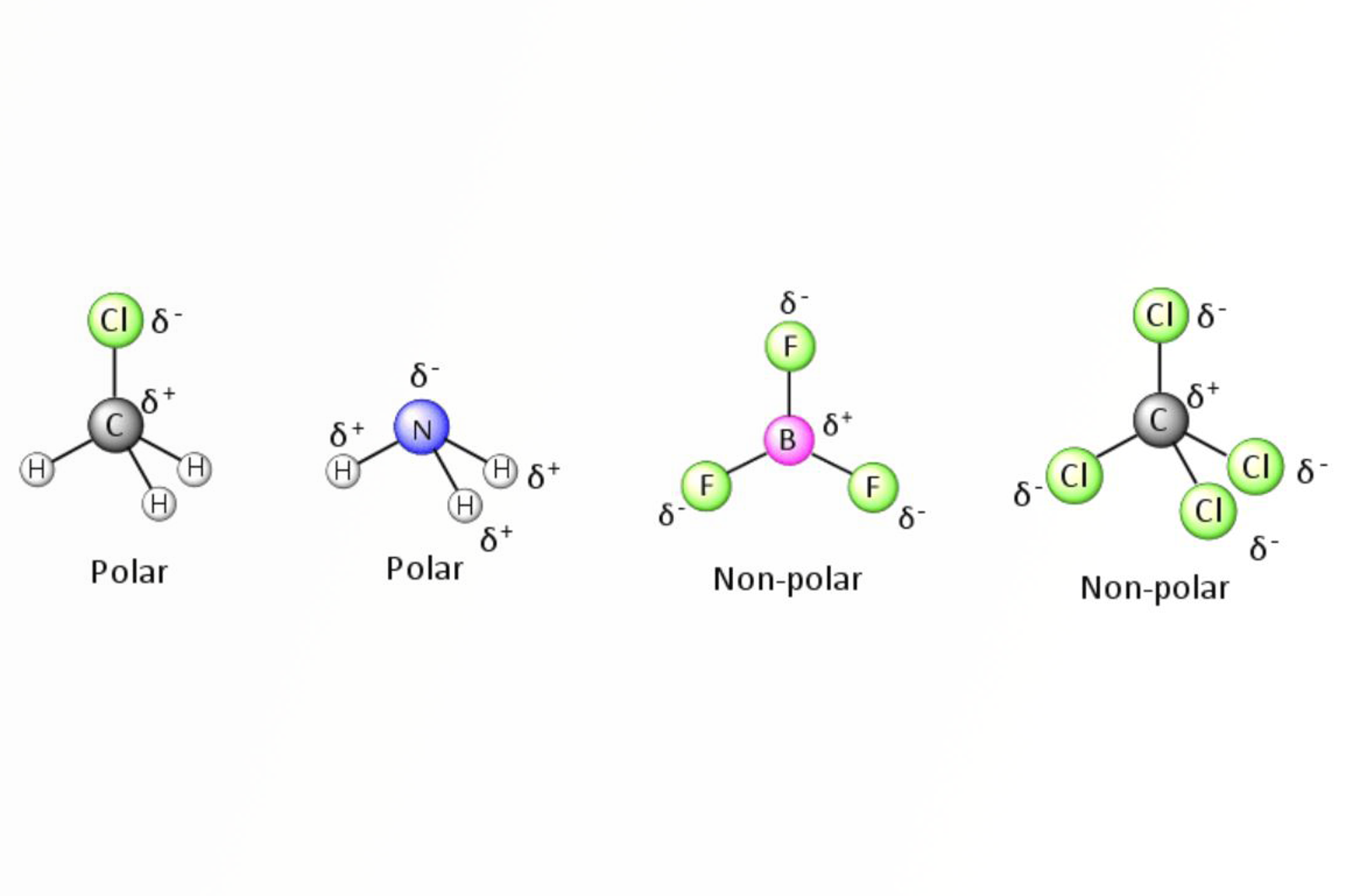
Analyte Chemistry for SPE
This quick guide discusses the first step in developing a solid phase extraction (SPE) method; understanding your analyte(s) chemistry.

QuEChERS Video Guide
This quick guide video provides you with the knowledge to get started with QuEChERS.
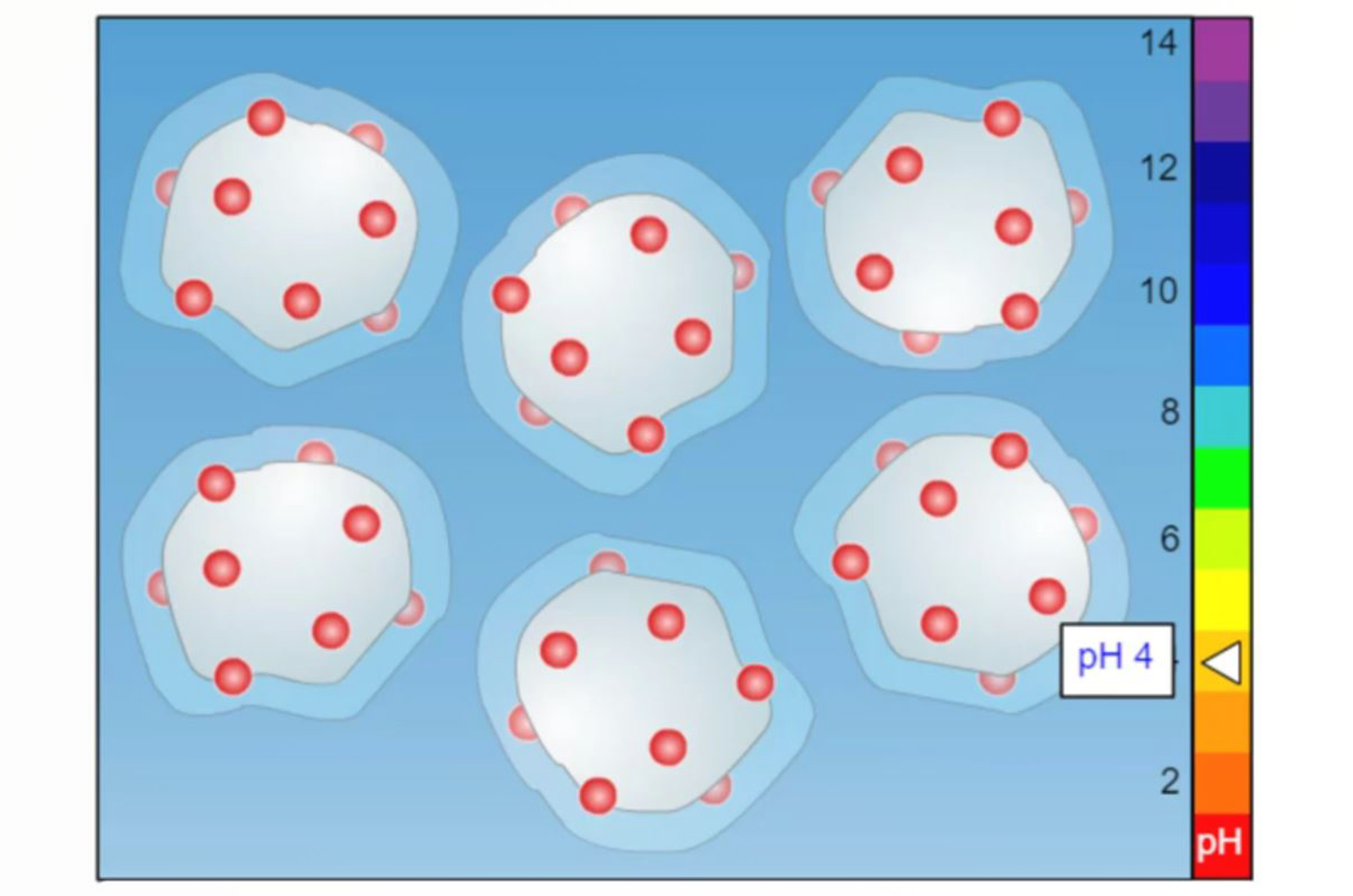
What Can SLE Do for Me?
An interesting and useful variation of liquid/liquid extraction (LLE) is supported liquid extraction (SLE). While the chemistry of this technique is very similar to that of LLE the physical nature of the technique offers distinct benefits which are detailed in this quick guide.
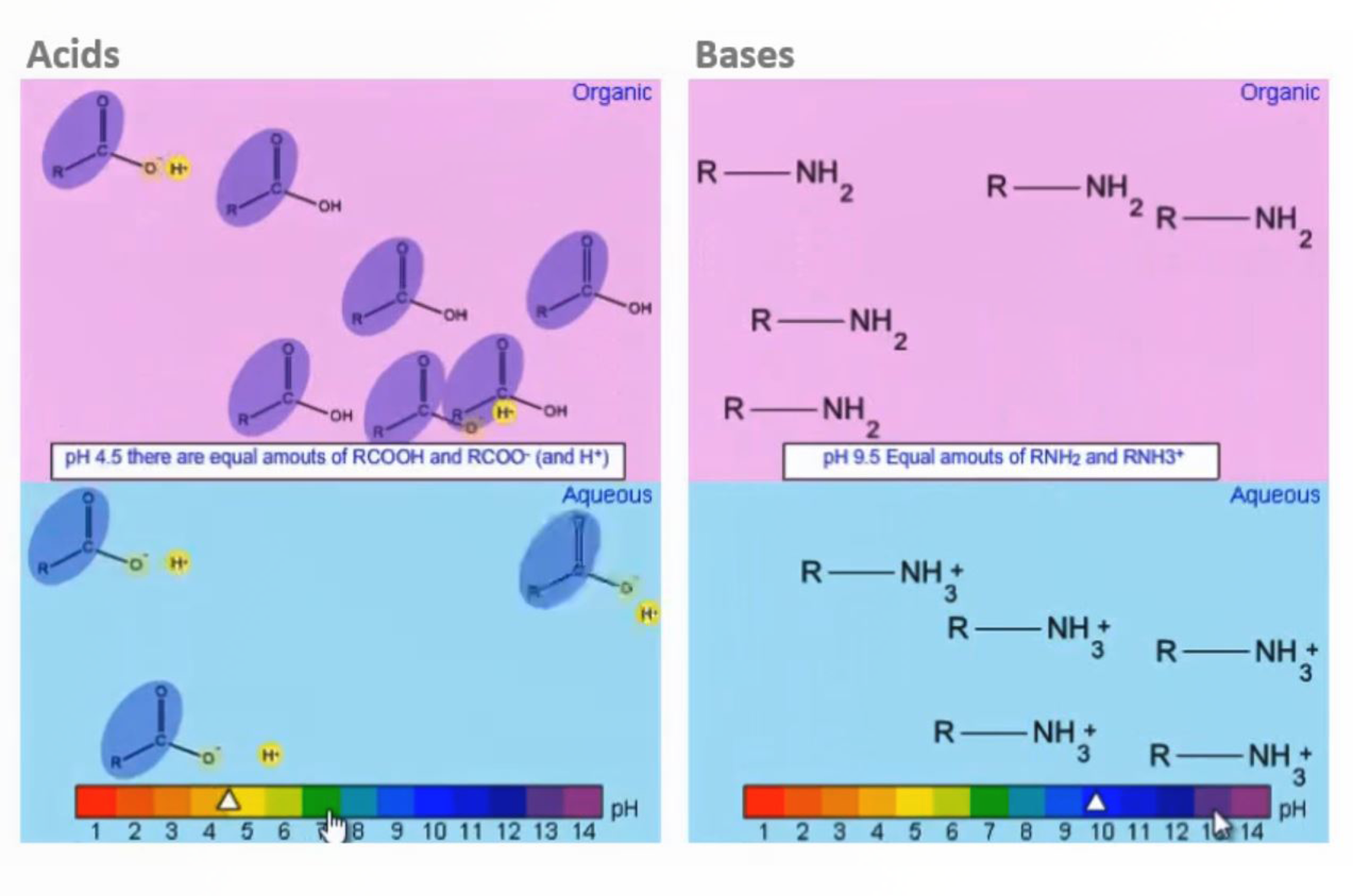
SPE - Molecular Properties: Ionic Groups
This video focuses on the importance of functional group chemistry in analytical science, including its impact on sample solvents, stationary phase selection, and analyte ionization in mass spectrometry. Understanding and identifying ionic functional groups in analytes is essential for optimizing analysis.
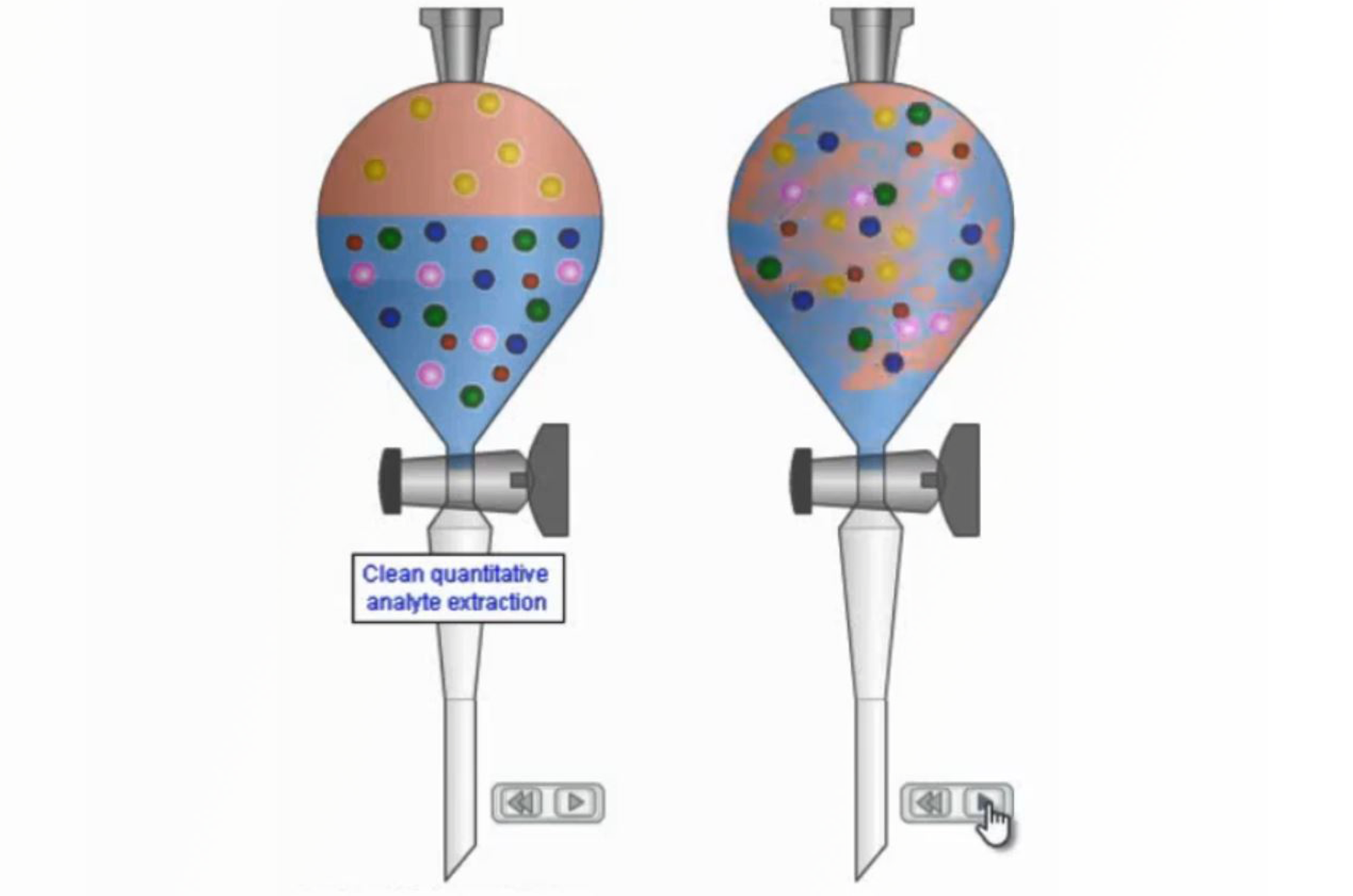
SPE Mechanisms - Polar SPE
This video will detail how the polar SPE extraction mechanism works, as well as, considering the individual protocol steps.
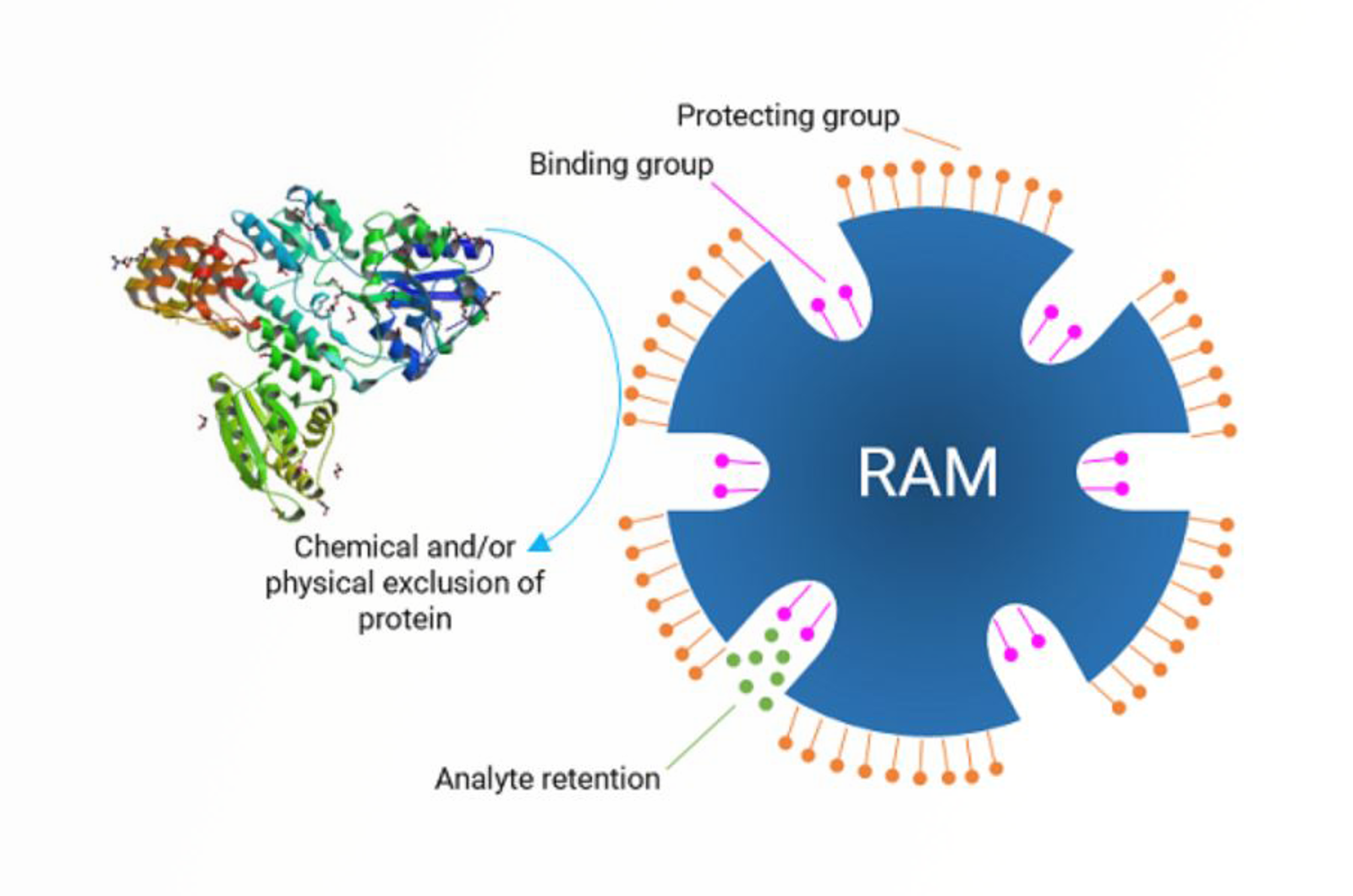
Modern SPE Sorbents
This quick guide focusses on modern SPE phases which have been created and used for specific applications which have proven problematic on standard SPE sorbents.
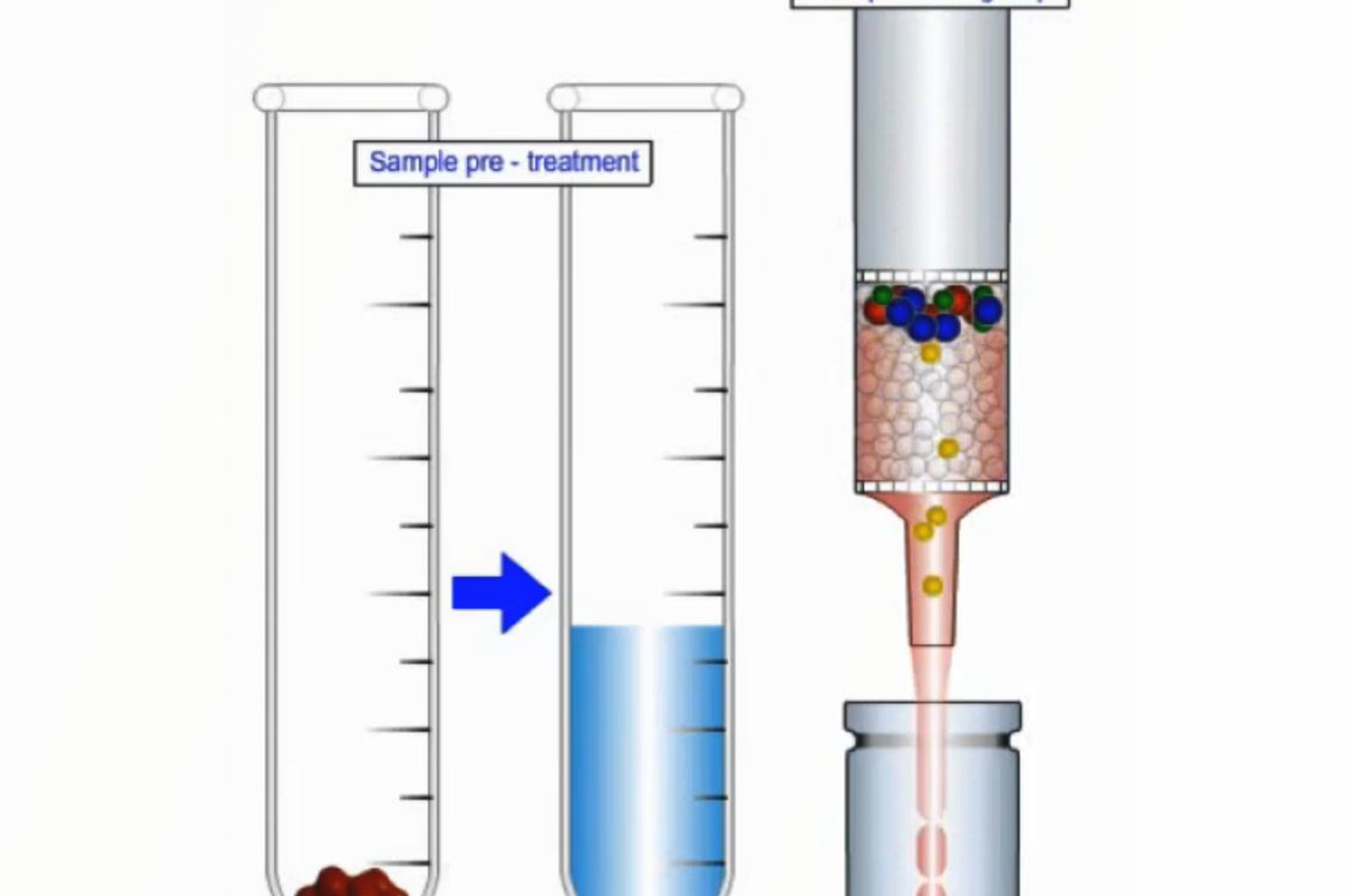
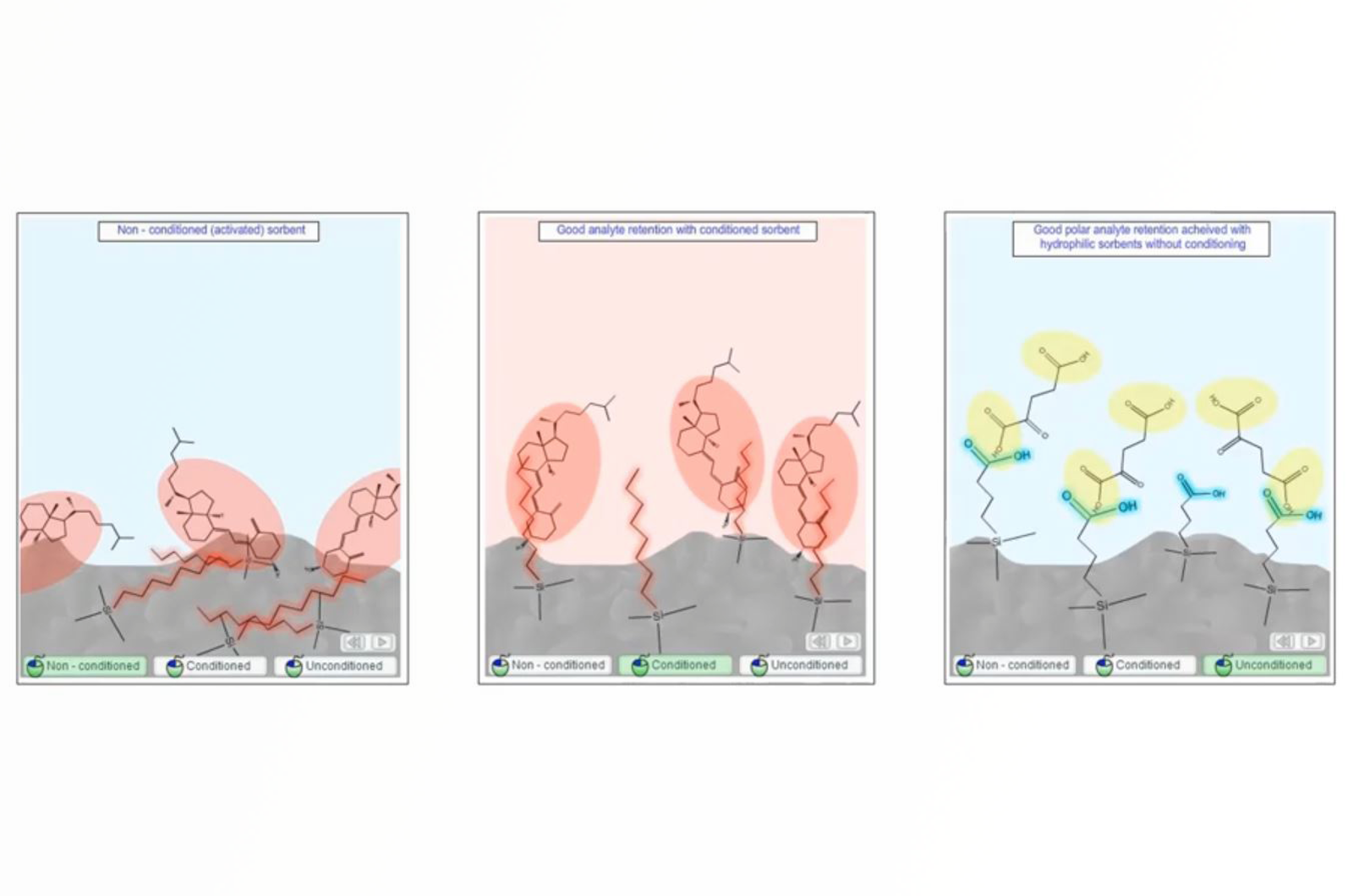
SPE - Column Conditioning
This video will discuss why we need to condition SPE sorbents, as well as, how this is achieved with the various modes of SPE employed.
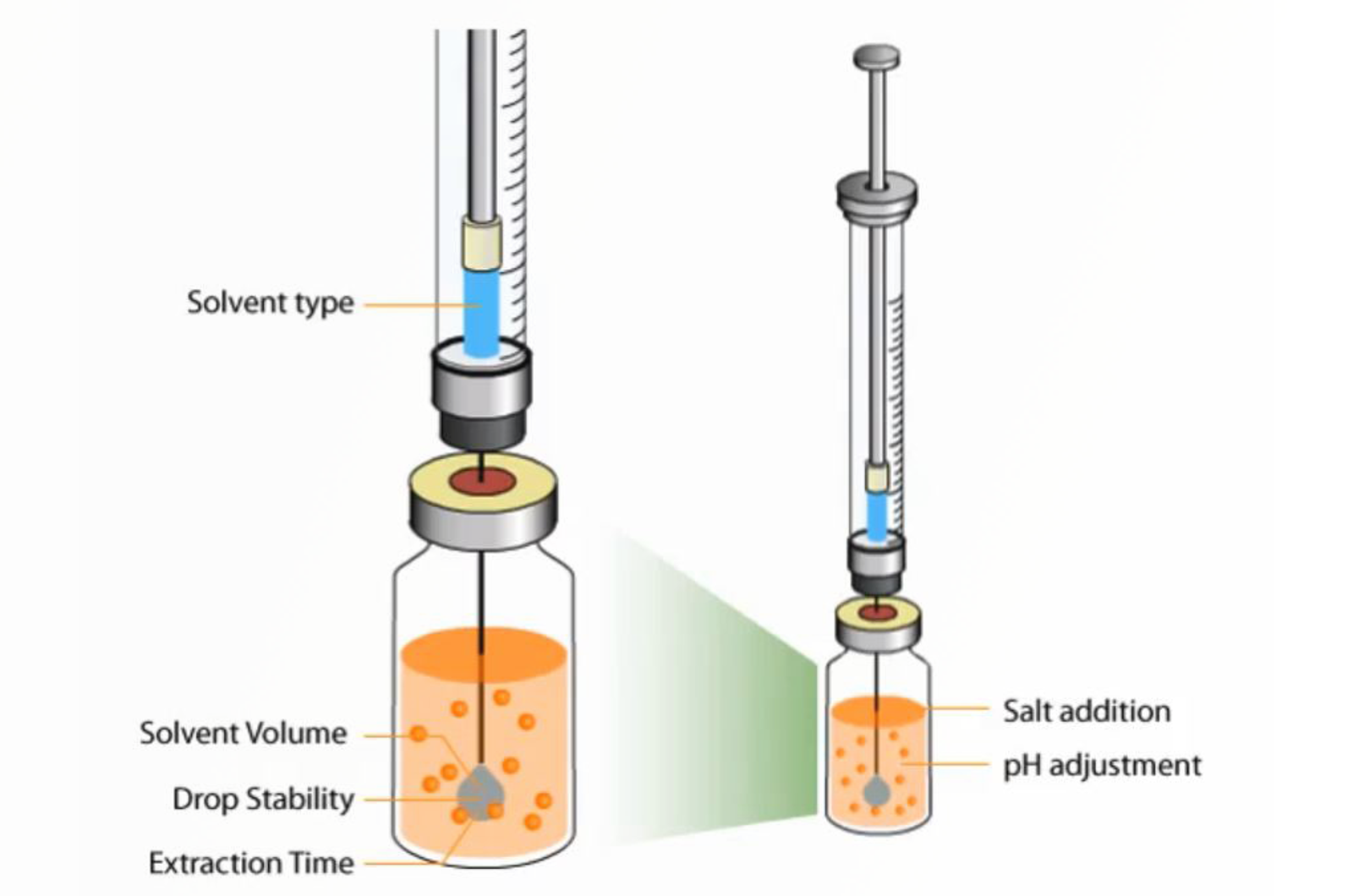
Sample Preparation - Microextraction: SDME
This short video will look at the theory and optimization of single drop microextraction (SDME).
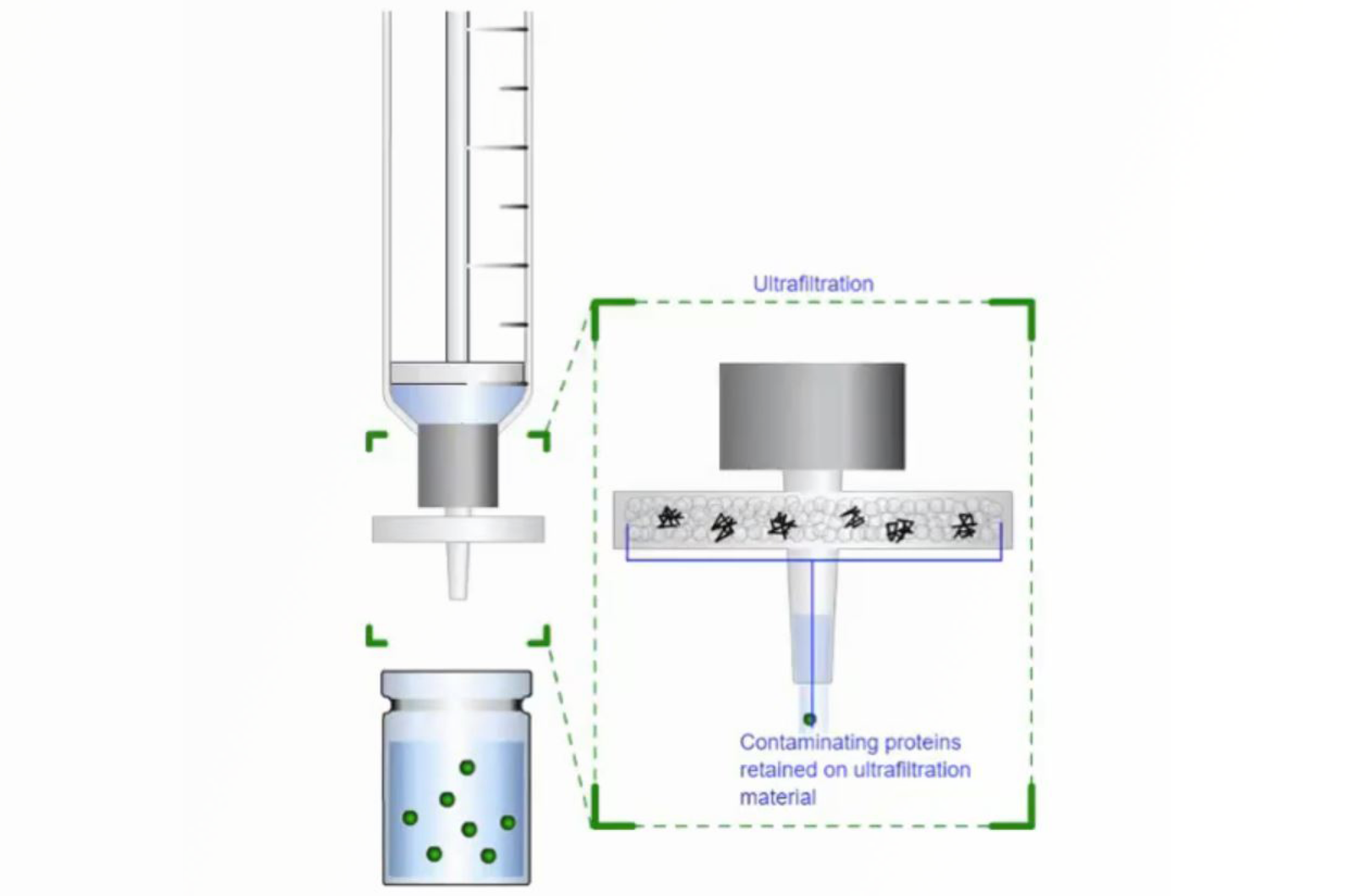
Everything You Need to Know About Filtration
This webcast will provide information on how to correctly select a filter for your application allowing you to avoid some of the common pitfalls related to filtration.
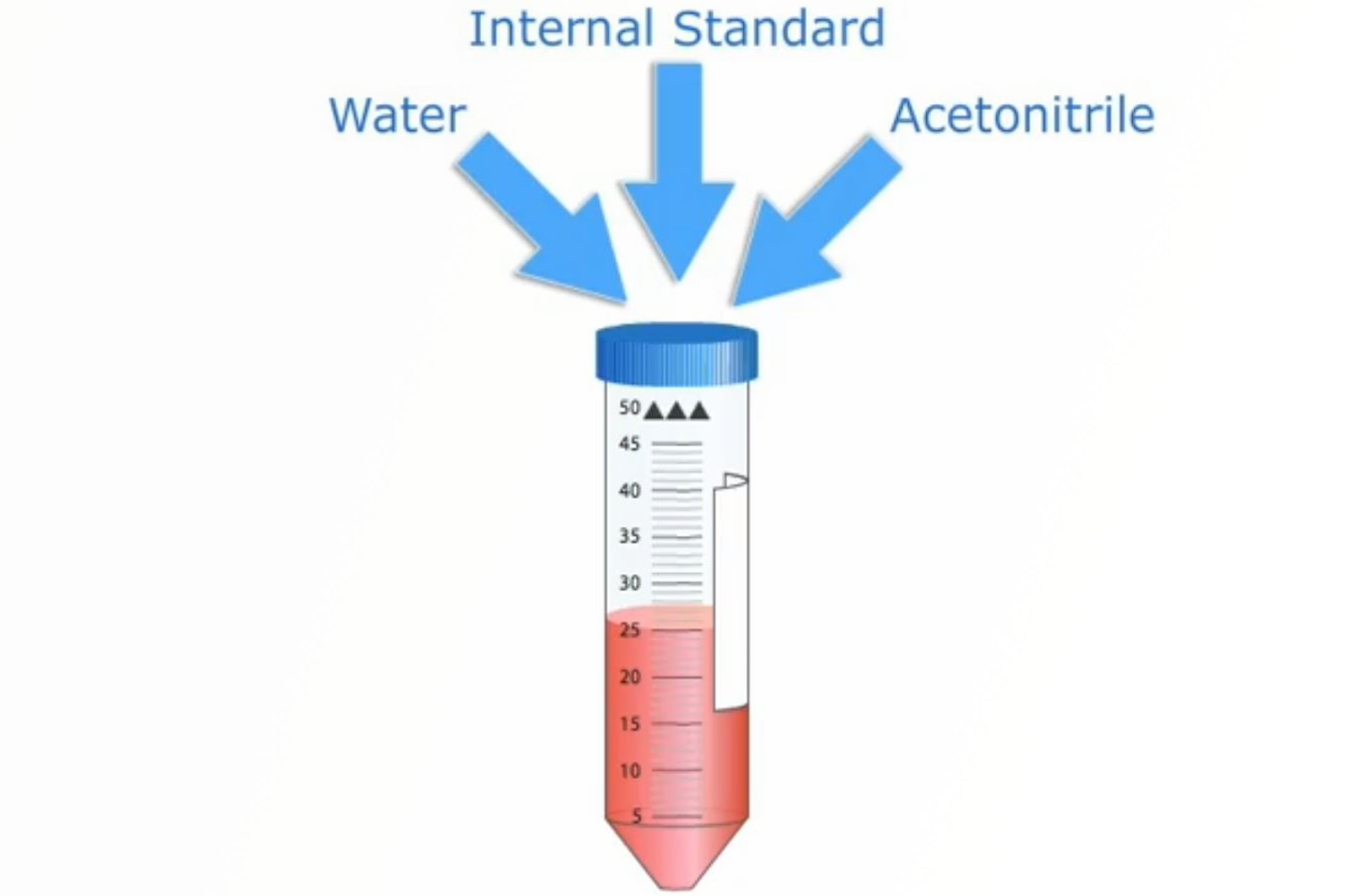
QuEChERS
This module will describe the underlying scientific principles behind QuEChERS allowing you to fully understand and successfully apply the process. The standard methodologies and the protocol steps are described in detail along with information on how to select a quenchers method for your application.

CHROMTalks Sample Preparation Series
Highly experienced speakers from industry and academia take some time to discuss their past (and more recent) experiences with chromatography and impart their key learnings. From heroic failures that have taught them valuable lessons, to moments of inspirational serendipity, and everything in between.
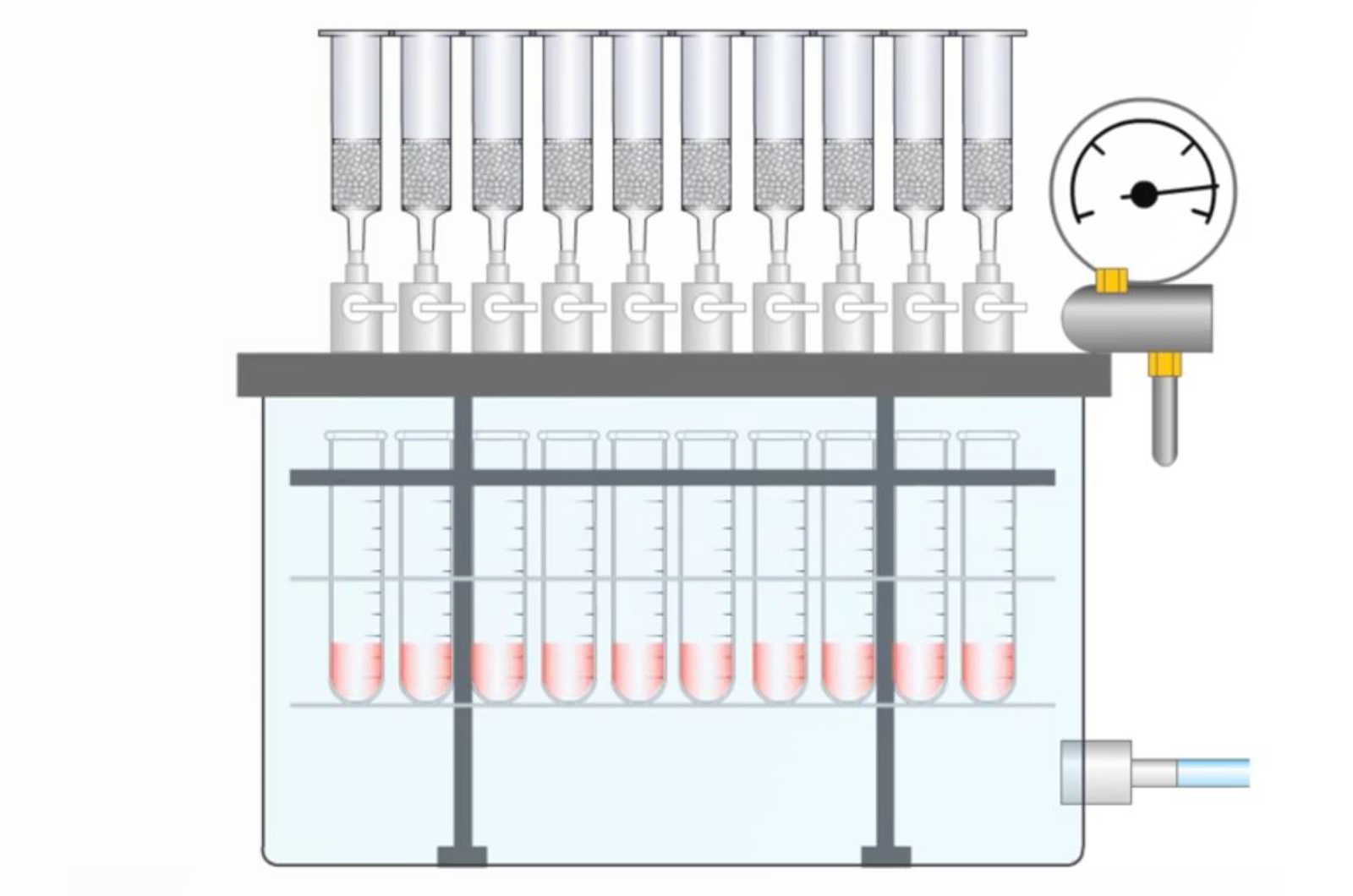
Vacuum or Positive Pressure: Which is Best for SPE Processing?
The simple task of processing the SPE columns or plates is not free of error, therefore, this article looks at the methods used for SPE processing and decides which is best.
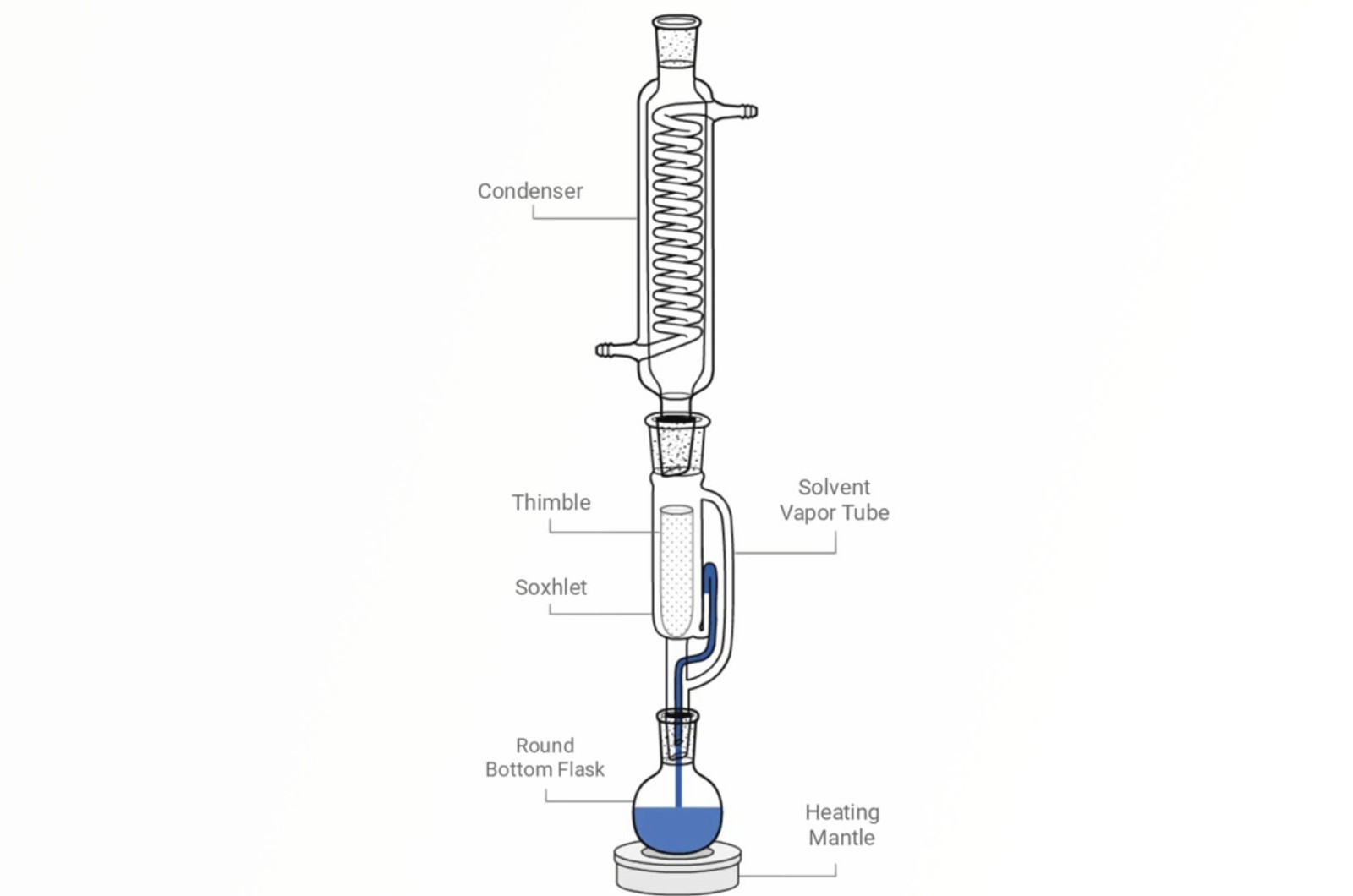
Soxhlet Extraction
This quick guide details how Soxhlet extraction works, parameters that require optimization, and how improvements to the methodology and automation have allowed an increase in throughput and improved reproducibility.
