Chromatography Training for Pharmaceutical Analysis
In the pharmaceutical industry, a variety of chromatographic analyses are used to ensure the quality, safety, and efficacy of drug products. These analyses are crucial for separating, identifying, and quantifying active pharmaceutical ingredients (APIs), excipients, impurities, degradation products, and contaminants. CHROMacademy provides in-depth training in LC-MS, GC-MS, and sample preparation techniques - empowering pharmaceutical analysts to deliver accurate, reproducible results.
Why Pharmaceutical Chromatography Training Matters
Proper training ensures analytical scientists and QA/QC teams can:
- Accurately detect and quantify active ingredients, impurities, and degradation products
- Manage complex matrices including formulations, biological fluids, and protein therapeutics
- Develop and validate robust, regulatory-compliant methods
- Increase lab efficiency and reduce variability in results
Whether you're working in quality control, R&D, or regulatory affairs, our training helps maintain the highest analytical standards.

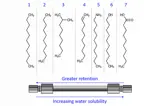
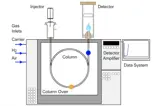
HPLC
HPLC is the most widely used chromatographic technique in the pharmaceutical industry for routine analysis.
Applications:
- Quantification of APIs and excipients in drug formulations
- Impurity profiling and testing for degradation products
- Stability studies of pharmaceutical products,
- Bioequivalence testing
- Quality control (QC) and release testing of drug products
- Analysis of active ingredients, excipients, preservatives, and contaminants in various drug forms (oral tablets, injectables, creams, etc.)
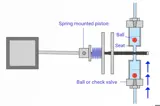
GC
GC is primarily used for the analysis of volatile and semi-volatile compounds.
Applications:
- Residual solvent testing in drug products (e.g., solvents used during synthesis or formulation)
- Analysis of volatile impurities in APIs
- Monitoring and quality control of volatile components like solvents or essential oils
- Determining the composition of volatile organic compounds (VOCs) in drug formulations and packaging
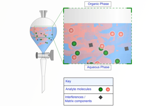
Sample Preparation
This course has been designed for those who are experience in GC and who are now looking to develop their experience and understanding of method development.
Applications:
- Purification of active pharmaceutical ingredients (APIs) and excipients,
- Removal of impurities or degradation products
- Sample clean-up in bioanalysis (e.g., blood, serum, or urine)
- Removal of matrix interference from biological samples prior to HPLC or GC analysis

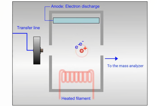
GC-MS
GC-MS is widely used in pharmaceutical analysis for the sensitive and specific identification and quantification of volatile and semi-volatile compounds, such as residual solvents and impurities.
Applications:
- Volatile compound analysis
- Residual solvent analysis
- Analysis of impurities
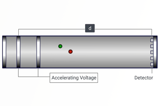
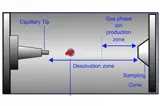
LC-MS
LC-MS is a powerful tool in pharmaceutical analysis for sensitive and specific detection of drugs, metabolites, and impurities in complex matrices.
Applications:
- Drug quantification metabolite identification
- Pharmacokinetics and bioavailability
- Impurity profiling
- Stability studies
Useful CHROMacademy Learning Paths for Pharmaceutical Analysts
Start Your Pharmaceutical Laboratory Training Today
Join global pharmaceutical professionals who trust CHROMacademy for expert-led, flexible, and compliant pharmaceutical analysis training. Whether you're optimizing HPLC for drug product analysis or learning mass spectrometry for impurity profiling, our platform supports your success.